Abstract
Active episodes of the inflammatory bowel diseases are associated with the infiltration of large numbers of myeloid cells including neutrophils, monocytes, and macrophages. The objective of this study was to systematically characterize and define the different populations of myeloid cells generated in a mouse model of chronic gut inflammation. Using the T cell transfer model of chronic colitis, we found that induction of disease was associated with enhanced production of myelopoietic cytokines (IL-17 and G-CSF), increased production of neutrophils and monocytes, and infiltration of large numbers of myeloid cells into the mesenteric lymph nodes (MLNs) and colon. Detailed characterization of these myeloid cells revealed three major populations including Mac-1+Ly6ChighGr-1low/neg cells (monocytes), Mac-1+Ly6CintGr-1+ cells (neutrophils), and Mac-1+Ly6Clow/negGr-1low/neg leukocytes (macrophages, dendritic cells, and eosinophils). In addition, we observed enhanced surface expression of MHC class II and CD86 on neutrophils isolated from the inflamed colon when compared with neutrophils obtained from the blood, the MLNs, and the spleen of colitic mice. Furthermore, we found that colonic neutrophils had acquired APC function that enabled these granulocytes to induce proliferation of OVA-specific CD4+ T cells in an Ag- and MHC class II-dependent manner. Finally, we observed a synergistic increase in proinflammatory cytokine and chemokine production following coculture of T cells with neutrophils in vitro. Taken together, our data suggest that extravasated neutrophils acquire APC function within the inflamed bowel where they may perpetuate chronic gut inflammation by inducing T cell activation and proliferation as well as by enhancing production of proinflammatory mediators.
Inflammatory bowel diseases (IBD; Crohn’s disease [CD] and ulcerative colitis) are multifactorial inflammatory disorders of the gastrointestinal tract that result from a complex interaction among genetic, environmental, and immune factors. Active episodes of the disease are associated with the infiltration of large numbers of leukocytes including lymphocytes, granulocytes, monocytes, and macrophages (1–3). This enhanced inflammatory infiltrate is thought to contribute to the intestinal injury and dysfunction resulting in abdominal pain, rectal bleeding, and diarrhea. Using mouse models of IBD, we and others (D.V. Ostanin, E. Kurmaeva, S.M. Berney, and M.B. Grisham, unpublished observations; 4–7) have demonstrated that in addition to expansion of colitogenic T cells, induction of chronic intestinal inflammation is associated with a large and significant increase in the numbers of myeloid cells bearing markers CD11b (Mac-1) and Gr-1 in the bone marrow (BM), circulation, mesenteric lymph nodes (MLNs), the spleen, and colonic lamina propria (cLP). Although T cells undoubtedly play an important role in the induction and perpetuation of disease, the role of myeloid cells in disease pathogenesis has not been defined clearly.
The accumulation of myeloid cells such as neutrophils, monocytes, and macrophages and others in the inflamed gut and their release of a variety of proinflammatory reactive oxygen and nitrogen species, cytokines, chemokines, and proteases are thought to contribute to tissue injury and disruption of the epithelial barrier. Indeed, neutrophil-associated markers are highly expressed in chronic active IBD (8), and the transepithelial migration of neutrophils into the bowel lumen to form crypt abscesses is a characteristic feature of active disease (9, 10). Despite these observations, no clear consensus has been reached from several different studies that have attempted to define the role of myeloid cells in the pathogenesis of intestinal inflammation. Although some studies have suggested that neutrophils or other myeloid cells promote inflammatory tissue injury (11, 12), other investigations were unable to confirm these findings (13, 14). Contributing even further to the confusion has been the observation that depletion of intestinal mononuclear phagocytes using clodronate–encapsulate liposomes worsened the colonic inflammation induced by dextran sulfate sodium. The enhanced inflammation and injury observed in this study was found to be associated with an increased neutrophil infiltration into the colon, suggesting a pathogenic role for these cells (12). In contrast to these studies, recent reports have demonstrated that depletion of neutrophils actually exacerbates colonic inflammation and the systemic wasting syndrome in chronic (5, 6) and acute colitis models (7), suggesting that neutrophils may function to suppress intestinal inflammation. The depletion of neutrophils in these studies used the in vivo administration of the anti–Gr-1 Ab (clone RB6-8C5) prior to or following induction of disease. Although this strategy has previously proven effective in depleting neutrophils in healthy mice (15), administration of this Ab to mice with inflammation is known to induce profound and lethal respiratory and cardiovascular complications that may have devastating systemic effects including death of the animal (16, 17). Another confounding variable that makes interpretation difficult is the fact that anti–Gr-1 Ab recognizes both Ly6G and Ly6C; thus, its administration would deplete not only neutrophils (bearing Ly6G on their surface) but also Ly6C+ myeloid cells with potent immunosuppressive properties (18, 19). A recent study that analyzed the effects of in vivo anti-Ly6G (clone 1A8) and anti–Gr-1 (RB6-8C5) Ab administration on blood neutrophils and monocytes confirmed that the latter significantly depleted Gr-1intF4/80+ monocytes (20).
Because of these experimental limitations and lack of a clear consensus as to the role of neutrophils and other myeloid cells in the pathogenesis of chronic gut inflammation, we undertook a systematic analysis and characterization of myeloid cell generation, phenotype, and function in a mouse model of chronic gut inflammation.
Materials and Methods
Animals
Wild-type (WT) mice, recombination activating gene-1–deficient (RAG−/−; B6.129S7-Rag1tm1Mom/J) mice, and OVA-specific B6.Cg-Tg (TcraTcrb)425Cbn/J (OTII) mice (6–8 wk of age) all on the C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were maintained on 12-h/12-h light/dark cycles in standard animal cages with filter tops under specific pathogen-free conditions in our animal care facility at Louisiana State University Health Sciences Center (Shreveport, LA) and given standard laboratory rodent chow and water ad libitum. All experimental procedures involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center and performed according to the criteria outlined by the National Institutes of Health.
Abs and reagents
Abs were purchased from either BD Pharmingen (San Diego, CA) or eBioscience (San Diego, CA). Negative selection kits for isolation of mouse CD4+ T cells were purchased from either Invitrogen (Carlsbad, CA) or StemCell Technologies (Vancouver, BC, Canada).
Induction of chronic gut inflammation
Chronic colitis was induced by the transfer of sorted WT CD4+CD45RBhigh T cells into RAG−/− mice using our previously published method (22). Briefly, spleens were removed from donor WT mice and teased into a single-cell suspension in 1× PBS/4% FBS (FACS buffer). CD4+ cells were obtained using negative selection kits, according to the manufacturer’s protocol. Enriched CD4+ T cells were labeled with anti-CD4 and anti-CD45RB mAb and sorted into CD45RBhigh (40% brightest) and CD45RBlow (15% dimmest) fractions using FACSAria (BD Biosciences, San Jose, CA) and were found to be >98% pure on postsort analysis. Male RAG−/− mice were injected (i.p.) with 5 × 105 CD45RBhigh, CD45RBlow, or both T cell subsets resuspended in 500 μl PBS. For some experiments, we adoptively transferred flow-purified CD4+CD25− T cells into RAG−/− recipients to induce colitis (5 × 105; i.p. injected). In general, colitis induced by transfer of CD4+CD25− T cells was comparable to that induced by CD45RBhigh T cells, although we did see a slight reduction in the incidence of moderate/severe disease in the CD4+CD25−→RAG−/− mice compared with the CD45RBhigh→RAG−/− mice. Clinical evidence of disease in mice (e.g., body weight loss and loose stool/diarrhea) was followed and recorded weekly from the time of the injection.
Histopathology
Eight weeks following T cell transfer or when the animals lost 15–20% of their original body weights, mice were euthanized, and colons were excised, measured, and weighed. Gross colitic scores were assigned according to previously published criteria (21). Representative pieces of proximal and distal colon were fixed in 10% phosphate-buffered formalin, sectioned, and stained with H&E. Blinded histopathological analysis of each colon was performed by a pathologist unaware of the identity of each sample using our previously published scoring criteria (21–23).
Quantification of circulating leukocytes, and serum cytokines
Blood was withdrawn from anesthetized mice at the time of sacrifice via the inferior vena cava using a 25 g needle attached to a 1-ml heparinized syringe. After 500 μl heparinized blood was mixed with an equal volume of 3% dextran (>400,000 m.w.) in PBS, the erythrocytes were allowed to settle for 30 min at room temperature, and leukocyte-rich supernatant was removed and stored on ice. Remaining erythrocytes in the supernatant were lysed, and leukocytes were pelleted by centrifugation, resuspended in PBS, and mixed with crystal violet dissolved in 3% acetic acid for counting using a hemocytometer. Total blood leukocytes were expressed as cells per milliliter of blood. For differential cell counts, 50,000–100,000 circulating or tissue-derived/flow-purified leukocytes were resuspended in 100 μl 1× PBS and dispensed into the cytospin chamber loaded with Colorfrost Plus Microscope Slides (Fisher Scientific) and cardboard filter (Thermo Electron) and centrifuged (600 rpm, 2 min) using Shandon Cytospin3 centrifuge. Following this, samples were air-dried for 1–2 min and stained using a Diff-Quick kit (Siemens Healthcare Diagnostics), according to the manufacturer’s instructions. The slides were mounted under coverslip using Permount mounting agent (Fisher Scientific). At least 200 total cells were counted from at least four to five different fields, and those with mononuclear or polymorphonuclear morphology were quantified and expressed as a percentage of the total cells counted.
For serum cytokine determinations, blood was withdrawn from anesthetized mice and allowed to clot for 30–60 min at room temperature. The blood was centrifuged (12,000 × g for 10 min) in BD Microtainer serum separator tubes, and the serum was removed and stored at −80°C. Serum GM-CSF and IL-17 were quantified using ELISA kits (eBioscience), according to the manufacturer’s instructions.
Myeloperoxidase assay
Representative pieces of colon were rinsed with ice-cold PBS, blotted dry, and immediately frozen with liquid nitrogen. The samples were stored at −80°C until thawed for myeloperoxidase (MPO) activity determination using the O-dianisidine method, as described previously (24, 25). Briefly, samples were thawed, weighed, and suspended (10% w/v) in 50 mM potassium phosphate buffer (pH 6), containing 0.5% hexadecyltrimethylammonium bromide buffer (0.1 g/20 ml potassium phosphate buffer), and homogenized using Brinkmann Homogenizer. A 1-ml sample of the homogenate was sonicated using a Labsonic 2000 generator and a Braun-Sonic 2000U transducer with a 40T needle probe tip (B. Braun Biotech International, Allentown, PA) twice for 15 s (approximate average power output: 15 W with continuous operation). The sample was then centrifuged at 12,000 × g for 10 min at 4°C, and the supernatant was saved on ice. The reaction was initiated by adding a small aliquot of supernatant (30 μl) to a prewarmed (to 37°C) reaction mixture containing 50 mM potassium phosphate (pH 6), O-dianisidine dihydrochloride (200 μg/ml), and hydrogen peroxide (200 μM). The reaction mixture was incubated for 5 min at 37°C, stopped by adding sodium azide (0.02% final concentration), and absorbance at 460 nm was determined using a spectrophotometer. The amount of MPO in the colon was expressed as the units of MPO per gram of tissue. One unit is defined as the amount of enzyme necessary to produce a change in absorbance of 1.0/min.
Tissue lymphocyte isolation and flow cytometry analyses
Lymphocytes were obtained from the spleen, the MLNs, and the cLP and analyzed by flow cytometry as described previously (22, 23, 26). Briefly, spleens were removed from reconstituted RAG−/− and teased into a single-cell suspension using frosted ends of two glass slides. RBCs were removed by hypotonic lysis, and the resulting leukocytes were washed and passed through 70-μm cell strainer to remove large chunks and then resuspended in FACS buffer. Cells from MLNs were obtained by grinding the tissue through a 70-μm nylon cell strainer (BD Falcon) in FACS buffer and further purified over 44/70% Percoll gradient. Lamina propria (LP) lymphocytes were prepared by digestion of finely minced intestinal pieces remaining after intraepithelial lymphocyte isolation with RPMI 1640 medium/4% FBS/2.5 mM CaCl2 and containing 150 U collagenase type VIII (Sigma-Aldrich) twice for 40 min each time in a 37°C orbital shaker. Lymphocytes were further enriched by centrifugation over a 44/70% Percoll gradient. For flow cytometry staining, ∼1 × 106 cells were placed in individual wells of a 96-well plate, incubated first with FcR block (CD16/CD32), and then stained with appropriate Ab mixtures. After the staining, cells were fixed for 20 min on ice in freshly prepared 2% ultrapure formaldehyde (Polysciences, Warrington, PA) and analyzed the next day on the FACSCalibur or BD LSR II (BD Biosciences). Flow cytometry data were analyzed using FlowJo software for PC (version 7.2.5; Tree Star, Ashland, OR).
Granulocyte and myeloid cell isolation, T cell proliferation assay, and cytokine determinations
A single-cell suspension was prepared from three to four pooled spleens and cLP cells and stained with anti–Mac-1 (CD11b), -Ly6C, and –Gr-1 (Ly6G/C) Ab mixture, followed by sorting of Mac-1+ cells into Ly6CintGr-1+ using FACSAria (BD). To obtain a highly enriched population of OTII CD4+ T cells, the spleens from OTII mice were prepared as described above, stained with PE-labeled Abs to CD11c, Mac-1, CD8α, and B220 and allophycocyanin-tagged anti-CD4 Abs, and sorted into CD4+[CD11c/Mac-1/CD8α/B220] −. A total of 50,000 OTII CD4+ T cells were cocultured with the indicated number of sorted neutrophils in the presence of 10 μg/ml OVA peptide (323–339; AnaSpec, San Jose, CA). Where indicated, anti-CD1d (clone 1B1) or anti-MHC class II (MHC-II) (M5/114.15.2) Abs (1 μg/ml final) were added according to previously published protocol (27). For fixation, sorted myeloid cells were fixed in culture medium containing 1% paraformaldehyde for 10 min at 37°C, followed by washing and additional 10-min incubations twice in 120 mM lysine as described by Brimnes et al. (28). At the end of incubations, cells were washed an additional three times in medium prior to culturing. Cells were allowed to proliferate for 72 h with the addition of 1 μCi [3H]thymidine for the last 8–18 h of culturing. Cells lysates were harvested onto fiberglass filters using semiautomated cell harvester (Brandel, Gaithersburg, MD), and radioactivity was quantified using a liquid scintillation counter (1409; Wallac).
For cytokine analysis, negatively selected CD4+ T cells (50,000) isolated from the spleens of WT mice were cocultured in triplicate with sorted neutrophils in a 96-well flat-bottom plates in the presence of plate-bound anti-CD3 and soluble CD28 (1 μg/ml final) Abs. At 48 h following the addition of the Abs, supernatants from replicate wells were pooled, aliquoted, and frozen at −80°C. Samples from two independent experiments were included for subsequent cytokine analysis. Multiplex cytokine analysis was carried out using Milliplex Mouse Cytokine/Chemokine Immunoassay (Millipore, Billerica, MA), according to the manufacturer’s instructions. Briefly, samples were thawed on ice and diluted using the recommended buffer. Undiluted as well as 1:2 and 1:4 diluted samples were used for cytokine measurements, and only those values that fell within the range of the standard curve were used in calculations. Samples were run on Bio-Plex Luminex instrument (Bio-Rad Laboratories, Hercules, CA). Data were analyzed using Miliplex Analyzer 3.1 xPONENT software (Millipore). Samples from repeat experiments were analyzed on the same run.
Neutrophils from the spleens and colons of colitic mice were obtained by cell sorting. Ly6G+ cells from the BM were obtained by positive selection using Ly6G-PE Ab and EasySep PE Positive Selection Kit (StemCell Technologies). Cells from peritoneal exudate were obtained 4–6 h after injection of 25 mg oyster glycogen type II resuspended in 0.5 ml 1× PBS by peritoneal lavage. Ly6G+ cells from peritoneal exudate were isolated by positive selection. Following positive selection, cells were processed for RNA isolation and RT-PCR. Cytospin was performed as described above.
RNA isolation and RT-PCR
Total RNA from neutrophils obtained as described above was isolated using RNeasy Mini kit (Qiagen, Valencia, CA), according to the manufacturer’s instruction, and treated with DNase I (Qiagen). RNA concentration and purity was assessed with NanoDrop ND-1000 spectrophotometer (Thermo Scientific), and 50 ng was reversely transcribed with qScriptTM cDNA SuperMix (Quanta Biosciences), according to the manufacturer’s protocol. PCRs were carried out in a 25-μl volume using 2720 Thermo Cycler (Applied Biosystems) using primers specific for β2-microglobulin and histocompatibility class II Ag A, α-chain (I-Aα). Primer pair sequences for I-Aα were obtained from Leibundgut-Landmann et al. (30) and for β2-microglobulin from the RTPrimerDB database (RTPrimerDB ID: 3584) (30). Amplicons of expected length were resolved on a 1.5% agarose gel. For densitometry measurements, ImageJ software (version 1.44p) (31) was used, and the intensity of I-Aα band for each sample was normalized to that of β2-microglobulin.
Statistics
Data are presented as mean ± SEM (SE of mean). The statistical significance between two groups was determined using two-sided Student t test. The statistical significance between more than two groups was evaluated using a one-way ANOVA. The statistical significance between selected groups was evaluated using the Dunnett post hoc test. A p value <0.05 was considered significant.
Results
Chronic colitis is associated with increased myelopoiesis and elevations of serum myelopoietic cytokines
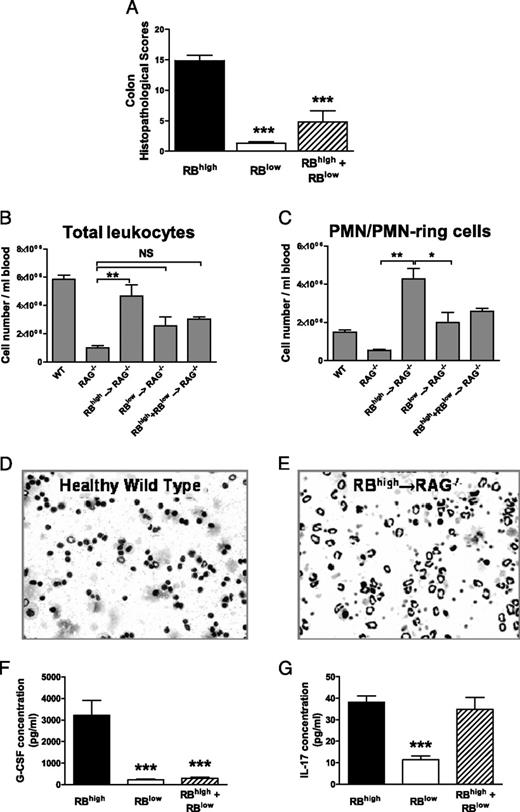
One of the hallmark features of chronic colitis induced in immunodeficient RAG−/− mice injected with naive (CD4+CD45RBhigh) T cells is the large and significant accumulation of myeloid cells (neutrophils, monocytes, and macrophages) within the cLP when compared with noncolitic RAG−/− mice injected with the CD45RBlow cells (CD45RBlow→RAG−/−) (Supplemental Fig. 1). In addition, this increased myeloid cell accumulation corresponds well with high levels of MPO activity in the cLP of colitic CD45RBhigh→RAG−/− mice compared with mice with little or no colitis (CD45RBlow→RAG−/−) demonstrating the presence of neutrophils, monocytes, and possibly eosinophils (Supplemental Fig. 1D). Development of chronic colitis (Fig. 1A) is accompanied by a 5- to 6-fold increase in circulating leukocytes compared with unmanipulated RAG−/− mice (Fig. 1B). Particularly striking was that the number of circulating neutrophils and PMN-ring cells increased ∼10-fold in colitic mice when compared with unmanipulated RAG−/− mice and 5-fold compared with WT mice (Fig. 1C). Morphologic analysis of peripheral blood obtained from colitic mice showed that the vast majority of circulating leukocytes had a multilobed or donut-shaped nucleus, which are morphological features of mouse granulocytes such as neutrophils and PMN-“ring cells” (Fig. 1E) (32, 33). PMN-ring cells represent newly released and not fully matured “band” neutrophils, whereas maturation and segmentation of their nucleus leads to the appearance of “segmented” neutrophils (34). Both types of neutrophils constituted >95% of the total circulating leukocytes in colitic mice (Fig. 1C, 1E).
Development of chronic colitis is accompanied by enhanced myelopoiesis and increase in peripheral granulocyte numbers. A, Blinded histopathological scores of colons obtained from CD45RBhigh→RAG−/−, CD45RBlow→RAG−/−, and CD45RBhigh+CD45RBlow→RAG−/− mice for at least 11 animals/group from at least two independently conducted experiments. B, Total leukocyte numbers in peripheral blood of mice from the different groups. Blood leukocyte counts in mice were determined as described in 1Materials and Methods. C, Total numbers of PMNs/PMN-ring cells in peripheral blood in mice. Representative images of peripheral blood cytospins from WT (D) and colitic CD45RBhigh→RAG−/− (E) mice showing dramatic increases in peripheral blood granulocytes. Serum from 7 to 11 individual mice/group was analyzed for G-CSF (F) and IL-17 (G). Data in all graphs shown represent the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Development of chronic colitis is accompanied by enhanced myelopoiesis and increase in peripheral granulocyte numbers. A, Blinded histopathological scores of colons obtained from CD45RBhigh→RAG−/−, CD45RBlow→RAG−/−, and CD45RBhigh+CD45RBlow→RAG−/− mice for at least 11 animals/group from at least two independently conducted experiments. B, Total leukocyte numbers in peripheral blood of mice from the different groups. Blood leukocyte counts in mice were determined as described in 1Materials and Methods. C, Total numbers of PMNs/PMN-ring cells in peripheral blood in mice. Representative images of peripheral blood cytospins from WT (D) and colitic CD45RBhigh→RAG−/− (E) mice showing dramatic increases in peripheral blood granulocytes. Serum from 7 to 11 individual mice/group was analyzed for G-CSF (F) and IL-17 (G). Data in all graphs shown represent the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Among the different cytokines responsible for stimulating myelopoiesis, we observed large and significant increases in serum G-CSF levels in colitic CD45RBhigh→RAG−/− mice when compared with either of the noncolitic CD45RBlow→RAG−/− or CD45RBhigh+CD45RBlow→RAG−/− groups (Fig. 1F). Serum level of IL-17 was significantly increased in colitic mice compared with the CD45RBlow→RAG−/− group (Fig. 1G) but was not significantly different from that in mice with attenuated disease (i.e., in CD45RBhigh+CD45RBlow→RAG−/− group) (Fig. 1F, 1G).
Phenotypic analysis of leukocytes derived from the colon, the MLNs, and the spleen of mice with chronic colitis
To more specifically define what different populations of leukocytes infiltrate animals with chronic disease, we analyzed leukocyte populations associated with the colon, the MLNs, and the spleen. Quantitatively, the numbers of CD4+ cells and Mac-1+Gr-1+ and Mac-1+Gr-1− cells in the colons were significantly higher in the colitic group compared with the noncolitic CD45RBlow→RAG−/− and CD45RBhigh+CD45RBlow→RAG−/− mice (Fig. 2A). We found that CD4+ T cells constituted a substantial portion (28%; range, 4–43%) of the total cells obtained from the inflamed colon. In addition, we observed significant numbers of granulocytes expressing both Mac-1 and Gr-1 (Mac-1+Gr-1+) as well as those that expressed Mac-1 but not Gr-1 (Mac-1+Gr-1−). Both populations combined represented ∼29% of total cLP cells (range, 15–42%) (Fig. 2B). We also observed large numbers of CD4+ T cells within the spleen and MLNs in the colitic CD45RBhigh→RAG−/− mice. In addition to T cells, Mac-1–expressing cells were elevated in the MLNs and spleens in mice with active disease. We observed large and significant increases in the absolute numbers of Mac-1+Gr-1+ and Mac-1+Gr-1− cells in the MLNs obtained from colitic when compared with their noncolitic controls (Fig. 2B). Although the numbers of Mac-1+Gr-1+ granulocytes were significantly increased in spleens from colitic mice compared with their noncolitic controls, the numbers of Mac-1+Gr-1− cells were similar among all three groups.
Enhanced accumulation of myeloid cells in mice with chronic colitis. A, Absolute numbers of CD4+ T cells, Mac-1+Gr-1+ and Mac-1+Gr-1- were quantified in CD45RBhigh→RAG−/− (black bars), CD45RBlow→RAG−/− (white bars), and CD45RBhigh + CD45RBlow→RAG−/− mice (hatched bars). At least five animals were analyzed per group and experiments were repeated at least twice. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the RBhigh control group. B, Representative dot plots showing percentages of Mac-1+Gr-1+ and Mac-1+Gr-1− cells in the spleens, MLNs, and colon LP. Numbers indicate percentages of cells within the gated leukocyte population (using forward and side scatter).
Enhanced accumulation of myeloid cells in mice with chronic colitis. A, Absolute numbers of CD4+ T cells, Mac-1+Gr-1+ and Mac-1+Gr-1- were quantified in CD45RBhigh→RAG−/− (black bars), CD45RBlow→RAG−/− (white bars), and CD45RBhigh + CD45RBlow→RAG−/− mice (hatched bars). At least five animals were analyzed per group and experiments were repeated at least twice. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the RBhigh control group. B, Representative dot plots showing percentages of Mac-1+Gr-1+ and Mac-1+Gr-1− cells in the spleens, MLNs, and colon LP. Numbers indicate percentages of cells within the gated leukocyte population (using forward and side scatter).
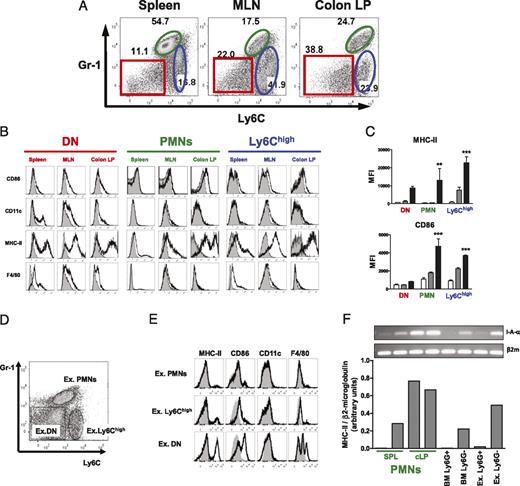
Identification of the different populations of murine myeloid cells by flow cytometry has been limited by the lack of specific markers. Mouse neutrophils and monocytes are commonly identified by flow cytometry using side scatter properties (granularity) and expression of Mac-1 and Gr-1 markers (36). Anti–Gr-1 Ab (clone RB6-8C5) recognizes both Ly6G, which is expressed primarily on mouse neutrophils (16), and Ly6C, which is expressed on a variety of cells, including T cells and monocytes (36, 37). Thus, Mac-1+Gr-1+ population can be further subdivided using anti–Gr-1 and anti-Ly6C Abs (19). Therefore, we next characterized the tissue-associated myeloid cells and identified three major populations, based on the expression of Mac-1 (CD11b), Gr-1(Ly6C/G), and Ly6C: Mac-1+Ly6ChighGr-1low/neg (hereafter referred to as Ly6Chigh), Mac-1+Ly6CintGr-1+ (Gr-1+) and Mac-1+Ly6Clow/negGr-1low/neg (double-negative, DN) (Fig. 3A). Gr-1+ population contains mature and immature neutrophils, and the Ly6Chigh population contains recently recruited immature monocytes (19, 38) that lose Ly6C expression following their maturation into tissue macrophages or DCs (39–42). The DN population consists of a heterogeneous mixture of mononuclear and granulocytic cells.
Increased expression of MHC-II and CD86 on PMNs isolated from inflamed colons. A, Dot plot analysis of cells isolated from spleen, MLNs, and colon LP showing expression of Ly6C versus Gr-1. Representative dot plots were initially gated on Mac-1+ (CD11b) cells. B, Representative histograms showing expression of indicated markers (black line) on Gr-1negLy6Cneg (DN), Gr-1+ PMNs, and Ly6Chigh populations. C, Median fluorescent intensity (MFI) values show progressive increase in MHC-II and CD86 expression on PMNs and Ly6Chigh cells isolated from cLP compared with those isolated from the spleen and MLN. **p < 0.01, ***p < 0.001. D, Expression of Ly6C and Gr-1 in peritoneal exudates cells. The dot plot was initially gated on Mac-1+ cells. Exudate PMNs (ex. PMNs) and Ly6Chigh and DN populations are shown by indicated gates. E, Histograms are gated on the corresponding populations identified in D. For all graphs, staining with Abs is indicated by black line, whereas staining with isotype control Ab is indicated by shaded curves. F, PMNs isolated from colitic mice show increased expression of MHC-II mRNA compared with PMNs obtained from the BM or peritoneal exudates. RT-PCR of histocompatibility class II Ag A α-chain and corresponding densitometry measurements. Gr-1+ PMNs from spleens and colons of colitic mice were obtained by cell sorting. Ly6G+ cells from the BM or peritoneal exudates were obtained by positive selection. Ly6G− fractions from BM (BM Ly6G−) and exudate (ex. Ly6G−) served as MHC-II+ control.
Increased expression of MHC-II and CD86 on PMNs isolated from inflamed colons. A, Dot plot analysis of cells isolated from spleen, MLNs, and colon LP showing expression of Ly6C versus Gr-1. Representative dot plots were initially gated on Mac-1+ (CD11b) cells. B, Representative histograms showing expression of indicated markers (black line) on Gr-1negLy6Cneg (DN), Gr-1+ PMNs, and Ly6Chigh populations. C, Median fluorescent intensity (MFI) values show progressive increase in MHC-II and CD86 expression on PMNs and Ly6Chigh cells isolated from cLP compared with those isolated from the spleen and MLN. **p < 0.01, ***p < 0.001. D, Expression of Ly6C and Gr-1 in peritoneal exudates cells. The dot plot was initially gated on Mac-1+ cells. Exudate PMNs (ex. PMNs) and Ly6Chigh and DN populations are shown by indicated gates. E, Histograms are gated on the corresponding populations identified in D. For all graphs, staining with Abs is indicated by black line, whereas staining with isotype control Ab is indicated by shaded curves. F, PMNs isolated from colitic mice show increased expression of MHC-II mRNA compared with PMNs obtained from the BM or peritoneal exudates. RT-PCR of histocompatibility class II Ag A α-chain and corresponding densitometry measurements. Gr-1+ PMNs from spleens and colons of colitic mice were obtained by cell sorting. Ly6G+ cells from the BM or peritoneal exudates were obtained by positive selection. Ly6G− fractions from BM (BM Ly6G−) and exudate (ex. Ly6G−) served as MHC-II+ control.
Ly6Chigh cells were identified in all tissues tested; however, they expressed varying levels of Gr-1: those isolated from the spleen had intermediate expression of Gr-1, whereas those from the colon LP were Gr-1low. In addition, all Ly6Chigh cells were SSClow and had morphology of monocytes (data not shown). Last, these cells did not express macrophage marker F4/80 and dendritic cells marker CD11c (Fig. 3B). Interestingly, MHC-II expression was very high on Ly6Chigh cells isolated from MLNs and colons but was low on cells isolated from spleens.
When we analyzed the Gr-1+ populations in all three tissues, we found that these cells were granular (SSChigh) and negative for both F4/80 and CD11c (Fig. 3B) and had the morphology characteristics of PMNs and PMN-ring cells (Supplemental Fig. 2A). We observed many fewer cells expressing a mature neutrophil morphology with a segmented nucleus within the Gr-1+ population isolated from the spleen when compared with MLNs and cLP. Furthermore, ∼75% of these cells displayed a ring-shaped morphology characteristic of immature “band” cells. In contrast, Gr-1+ cells isolated from the cLP were mostly mature “segmented” neutrophils (Supplemental Fig. 2B).
Analysis of the DN cells in the spleen revealed a heterogeneous population of cells: CD11chighF4/80+, CD11cintF4/80high, CD11cnegF4/80low, and CD11cnegF4/80neg (Supplemental Fig. 3). All cells within the CD11chighF4/80+ population were SSClow and MHC-IIhigh, which is consistent with the profile for DCs. Cells bearing CD11clowF4/80high phenotype were also SSClow and MHC-IIhigh, which is consistent with a phenotype of macrophages. In contrast, CD11cnegF4/80low cells contained large granular cells, based on their forward and side scatter profiles with intermediate expression of MHC-II. When DN cells were sorted and differentially stained (using Diff-Quick kit), some of these cells showed the cytoplasmic morphology and staining characteristics (red/pink granules) of eosinophils (Supplemental Fig. 2A). Indeed, low-level expression of F4/80 in combination with Mac-1 expression is characteristic of eosinophils (43). Splenic CD11cnegF4/80neg cells were SSClow with intermediate expression of MHC-II. These cells had morphology typical of a monocyte, with a bean-shaped nucleus or a monocyte-ring cell with donut-shaped nucleus (Supplemental Fig. 2A) (33). In addition, all of these cells were SSClow (Supplemental Fig. 3). DN population in the MLN or cLP was more homogeneous than that in the spleen. In the MLN, this population contained mainly CD11cnegF4/80low cells. Side scatter and MHC-II expression revealed two populations that were SSChighMHC-IIlow (possibly eosinophils) and SSClowMHC-IIhigh, which appear to be monocytes and macrophages. In the cLP, the same two populations were found within the DN gate: eosinophils (SSChighMHC-IIlow) based on the cytospin analysis and macrophage-like (SSClowMHC-IIhigh) cells that, interestingly, did not express a classical marker of mature macrophages F4/80 (44). However, it is known that under certain inflammatory conditions F4/80 can be downregulated by mature macrophages, especially in the T cell-rich areas (45). Because Arnold et al. (42) demonstrated that Ly6C, which is expressed at high levels on circulating monocytes, is downregulated upon their maturation into tissue macrophages, the SSClowMHC-IIhigh population most likely represents either mature macrophages or Ly6Clow-neg monocytes that can be found in the injured tissues (46).
Gr-1+ neutrophils obtained from the inflamed colon express enhanced levels of MHC-II and CD86
Neutrophils isolated from patients with chronic inflammatory conditions have been documented to undergo “transdifferentiation” into DC-like cells, that is, they acquire the ability to act as APCs and induce T cell activation (47–49). In addition, culturing human or mouse neutrophils with proinflammatory cytokines induces expression of MHC-II and costimulatory molecules (50–53) and delays apoptosis (54–56). Collectively, these reports suggest that during chronic inflammation neutrophils undergo phenotypic and functional changes driven, in part, by the inflammatory cytokines released by T cells. To assess whether neutrophils obtained from tissues of colitic mice assume APC-like properties, we analyzed surface expression of MHC-II and CD86 on these cells. We found that Gr-1+ neutrophils isolated from the spleen, the MLNs, and the cLP expressed low, moderate, and high levels of MHC-II, respectively (Fig. 3B). Because we established that Gr-1+ cells were negative for CD11c and F4/80, MHC-II expression could not be due to contaminating DCs or macrophages. We also observed a modest upregulation of CD86 with the highest levels seen on cells isolated from the inflamed cLP (Fig. 3B). Comparison of median fluorescence intensity between neutrophils isolated from different tissues showed that cLP cells had a significantly elevated expression of MHC-II and CD86 compared with those isolated from the spleen (Fig. 3C), suggesting that cells that infiltrated colons acquired phenotype of APC-like cells. We also analyzed expression of MHC-II and CD86 on DN and Ly6Chigh subsets of myeloid cells from the different tissues and observed enhanced surface expression of MHC-II on these cells (Fig. 3C). In general, pattern of CD86 expression paralleled that of MHC-II for both the DN and Ly6Chigh populations. Next, we wanted to determine whether MHC-II upregulation can be observed on neutrophils recruited in response to an acute inflammatory stimulus. To do this, we analyzed peritoneal exudate cells 4–6 h following the injection of oyster glycogen (Fig. 3D) (57). We found that these neutrophils had little surface expression of MHC-II or CD86 (Fig. 3E).
Whale et al. (58) reported that neutrophils may passively acquire parts of plasma membranes from apoptotic or necrotic cells during phagocytosis. Thus, high expression of MHC-II may be due to trogocytosis by neutrophils of this molecule from the surface of neighboring APCs. To evaluate whether Gr-1+ neutrophils actively transcribe MHC-II gene, we analyzed mRNA level of MHC class II in sorted neutrophils isolated from the spleen and the colon. We saw high levels of MHC-II Ag A α-chain mRNA in neutrophils obtained from cLP (Fig. 3F). In comparison, Ly6G+ neutrophils isolated from the BM showed no MHC-II mRNA. Interestingly, Ly6G+ peritoneal exudates neutrophils showed some expression, but it was much less than Ly6G− cells, which consist of a mixed population of various hematopoietic cells. These data suggest that recruitment of circulating neutrophils into the site of inflammation may provide transcriptional signals for the upregulation of MHC-II mRNA expression. These results confirmed that surface MHC-II expression in neutrophils isolated from mice with colitis is not due to trogocytosis but due to the transcription of mhc-II genes.
Neutrophils induce T cell activation through an Ag-specific and MHC-II–dependent interaction
To determine whether neutrophils isolated from colitic mice could induce proliferation of T cells, we cocultured these cells with flow-purified OVA-specific CD4+ T cells (OTII CD4+ cells) in the presence of OVA peptide. In our preliminary experiments, we found that positively or negatively selected splenic OTII CD4+ cells extensively proliferated in the presence of OVA peptide without addition of accessory cells (data not shown), suggesting that these population contained APCs. Thus, to remove CD4+ DCs present in the spleen (59) as well as other contaminating APCs, we sorted splenic OTII cells into CD4+[CD11c/Mac-1/CD8α/B220]neg cells. Only by sorting we were able to obtain a pure population of CD4+ T cells to use in our coculture experiments that did not proliferate in the presence of peptide without the addition of accessory cells (Fig. 4A). We found that neutrophils obtained from mice with active colitis in the presence of OVA peptide induced OTII T cell proliferation in a cell number-dependent manner (Fig. 4A). Interestingly, at a 1:1 ratio of neutrophils to T cells, those isolated from colon induced a 2-fold higher proliferation than those isolated from the spleen, which correlated with their higher surface expression of MHC-II. Omission of APCs or addition of MHC-II blocking Ab completely inhibited Ag-induced proliferation of T cells (Fig. 4B). The importance of Ag processing and presentation by cLP neutrophils was confirmed by formalin fixation of these cells, which abolished their ability to trigger proliferation (Fig. 4B). Taken together, these experiments suggest that Ag-specific proliferation of T cells by neutrophils isolated from colitic mice is dependent on their ability to internalize Ag and present it on their surface and is not due to nonspecific binding of Ag to the surface of these cells.
PMNs isolated from colitic mice induce proliferation of CD4+ T cells in an Ag-specific, MHC-II–dependent manner. A, PMNs isolated from colitic mice can function as APC and trigger proliferation of Ag-specific T cells. FACS-sorted OTII CD4+ T cells were cocultured with increasing numbers of sorted PMNs from spleens (white bars) or cLP (black bars) of colitic mice or WT iAccessory cells (hatched bars) in the presence of OVA peptide (10 μg/ml). *p < 0.05, **p < 0.01 compared with WT iAccessory cells. B, Ag-specific T cell proliferation is abolished with the addition of MHC-II blocking Ab but not in the presence of control anti-CD1d Ab. Fixation of PMNs or WT iAccessory cells also abolishes their ability to stimulate proliferation of T cells. Data are expressed as the mean cpm ± SEM from a representative experiment, which was repeated three to five times.
PMNs isolated from colitic mice induce proliferation of CD4+ T cells in an Ag-specific, MHC-II–dependent manner. A, PMNs isolated from colitic mice can function as APC and trigger proliferation of Ag-specific T cells. FACS-sorted OTII CD4+ T cells were cocultured with increasing numbers of sorted PMNs from spleens (white bars) or cLP (black bars) of colitic mice or WT iAccessory cells (hatched bars) in the presence of OVA peptide (10 μg/ml). *p < 0.05, **p < 0.01 compared with WT iAccessory cells. B, Ag-specific T cell proliferation is abolished with the addition of MHC-II blocking Ab but not in the presence of control anti-CD1d Ab. Fixation of PMNs or WT iAccessory cells also abolishes their ability to stimulate proliferation of T cells. Data are expressed as the mean cpm ± SEM from a representative experiment, which was repeated three to five times.
Coculture of Gr-1+ neutrophils with activated CD4+ T cells enhances the production of proinflammatory cytokines
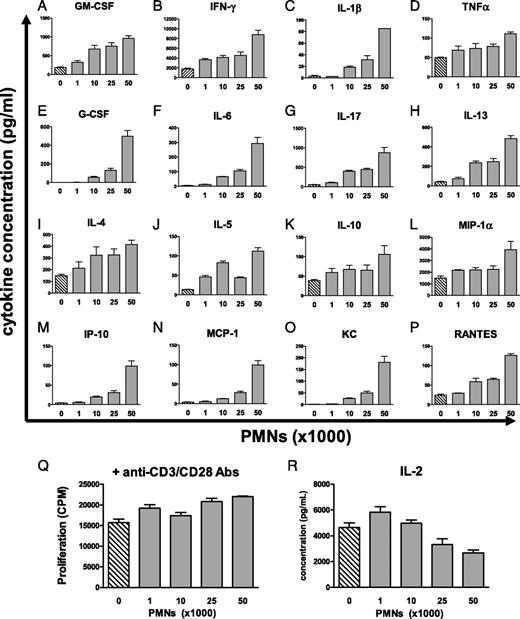
We next wished to ascertain whether neutrophils were capable of modulating the production of cytokines by T cells. We found that addition of increasing numbers of neutrophils isolated from the cLP to activated WT CD4+ T cells resulted in a cell number-dependent increases in the levels of proinflammatory mediators including IFN-γ, IL-17, TNF-α, and IL-1β (Fig. 5A–P). Increased production of cytokines that are thought to be important for myelopoiesis (e.g., IL-17, GM-CSF, G-CSF, and IL-6) was also observed with the addition of more neutrophils. We also observed a modest increase in production of Th2 cytokines such as IL-4 and IL-5 when ratios of neutrophils:T cells approached 1:1. Concentrations of several chemokines, such as IFN-γ–induced protein 10 (IP-10 or CXCL10), monocyte chemotactic protein-1 (MCP-1 or CCL2), regulated upon activation, normal T-cell expressed, and secreted (RANTES or CCL5), macrophage inflammatory protein-1α (MIP-1α or CCL3), and the chemokine KC (GRO-α or CXCL1) also increased in a cell-dependent manner. It should be noted that there were no significant increases in T cell proliferation and even a slight decrease in IL-2 concentrations at a 1:1 ratio of neutrophils:T cells (Figs. 5Q, 5R), suggesting that higher levels of cytokines in culture media cannot be due to the presence of more T cells. Slightly lower IL-2 levels with the addition of more neutrophils could be indicative of the inhibition of T cell proliferation despite the lack of any apparent effects on T cell proliferation as measured by the incorporation of [3H]thymidine (Fig. 5R).
PMNs synergize with T cells to enhance in cytokine production without an effect on proliferation. A–P and R, Coculturing of WT CD4+ T cells with PMNs isolated from cLP of colitic mice results in increased production of cytokines, and chemokines WT CD4+ T cells were cultured along (cross-hatched bars) or with the indicated number (shown on x-axis) of FACS-sorted PMNs (gray bars) in the presence of activating anti-CD3/CD28 Abs for 48 h. Cytokine concentrations were measured using multiplex cytokine analysis. Data are expressed as the mean ± SEM of serial dilutions (undiluted, 1:2 and 1:4) of each sample. Shown are results from a representative experiment of two independently conducted coculture experiments with similar results. Proliferation of WT T cells in response to anti-CD3/CD28 Abs in the absence or presence of PMNs (Q) was not substantially increased with the addition of PMNs.
PMNs synergize with T cells to enhance in cytokine production without an effect on proliferation. A–P and R, Coculturing of WT CD4+ T cells with PMNs isolated from cLP of colitic mice results in increased production of cytokines, and chemokines WT CD4+ T cells were cultured along (cross-hatched bars) or with the indicated number (shown on x-axis) of FACS-sorted PMNs (gray bars) in the presence of activating anti-CD3/CD28 Abs for 48 h. Cytokine concentrations were measured using multiplex cytokine analysis. Data are expressed as the mean ± SEM of serial dilutions (undiluted, 1:2 and 1:4) of each sample. Shown are results from a representative experiment of two independently conducted coculture experiments with similar results. Proliferation of WT T cells in response to anti-CD3/CD28 Abs in the absence or presence of PMNs (Q) was not substantially increased with the addition of PMNs.
Expression of cell adhesion molecules on neutrophils obtained from tissues of colitic mice
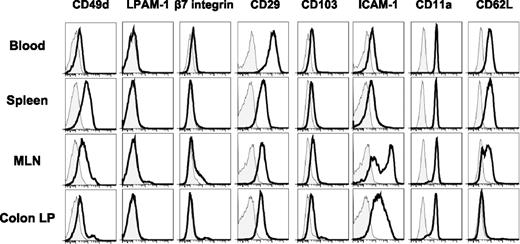
The large majority of studies investigating the molecular determinants involved in leukocyte trafficking in chronic gut inflammation have focused on T and/or B cell-associated adhesion molecules. Given our data demonstrating that the inflamed MLNs and colon contain large numbers of neutrophils, we wished to analyze surface expression of the major cell adhesion molecules on these cells found in blood and the different tissues in colitic mice. We found that expression of the α4 integrin was dramatically increased on neutrophils isolated from the spleen and the MLNs (Fig. 6). Because we did not observe any detectable expression of α4β7 (lymphocyte Peyer’s patch adhesion molecules-1) or β7 integrin, we concluded that expression of α4 on neutrophils from the spleen and the MLNs was largely due to the expression of α4β1. We did find that CD29 (β1 integrin) was highly expressed on circulating and tissue neutrophils obtained from mice with active colitis. Interestingly, the highest level of CD29 expression was seen on blood neutrophils where α4 expression was barely detectable. Because the β1 chain can pair with seven different α subunits (α1–6 and αV) to form VLA(1–6) and αVβ1 integrins, respectively (60), our data would imply that β1 integrins other than VLA-4 may be playing a role in recruitment of neutrophils from the blood. Not surprising, we found that the β2 integrins CD11a (Fig. 6) and CD11b (data not shown but was used for gating of CD11b+ cells in histograms shown in Fig. 6) were expressed on neutrophils isolated from blood, spleen, MLN, and cLP. The expression of the αE integrin (CD103) on neutrophils was relatively low, which suggested that αEβ7 expression is minimal. In addition to the different integrins, we found that neutrophils obtained from the blood, the spleen, and the MLNs but not cLP were L-selectin positive (Fig. 6). We also observed significant expression of ICAM-1 on neutrophils obtained from MLNs and cLP, whereas its expression on circulating and splenic neutrophils was somewhat reduced (Fig. 6). ICAM-1 expression by APCs is critical for stabilization of immunological synapse and efficient priming of T cells (61). Thus, enhanced ICAM-1 expression by MLN and cLP neutrophils provides additional evidence for their acquisition of Ag-presenting capacity.
Surface expression of different adhesion molecules on PMNs isolated from colitic mice. Representative histograms depicting expression of the indicated surface molecules (black line) were gated on PMNs (Mac-1+Ly6CintSSChighLy6G+) isolated from blood, spleen, MLNs, or cLP. Staining with fluorescently-tagged isotype control Ab is depicted by shaded histogram plots.
Surface expression of different adhesion molecules on PMNs isolated from colitic mice. Representative histograms depicting expression of the indicated surface molecules (black line) were gated on PMNs (Mac-1+Ly6CintSSChighLy6G+) isolated from blood, spleen, MLNs, or cLP. Staining with fluorescently-tagged isotype control Ab is depicted by shaded histogram plots.
Discussion
Histopathologic inspection of intestinal biopsies obtained from patients with active Crohn’s disease or ulcerative colitis reveal the presence of large numbers of myeloid cells such as neutrophils, monocytes, eosinophils, and macrophages (1–3). In addition, several studies using clinical specimens obtained from patients with active IBD have demonstrated increased levels of intestinal IL-8, G-CSF, and GM-CSF (62–64) that corresponded to a delay in neutrophil apoptosis (62). Because myeloid cells are capable of generating large amounts of proinflammatory mediators and reactive oxygen and nitrogen metabolites, investigators have proposed that these cells may play an important role in the inflammatory tissue injury observed in IBD (65–69). Indeed, transepithelial migration of neutrophils from the gut to the lumen to form crypt abscesses correlates with the worsening of disease (9, 10). Unfortunately, no clear consensus has been reached from a variety of different experimental and clinical studies attempting to define the importance of myeloid cells in the pathogenesis of IBD. As a first step toward understanding the role that myeloid cells may play in the induction, perpetuation, and/or resolution of chronic intestinal inflammation, we undertook a systematic analysis and detailed characterization of myeloid cell generation, phenotype, and function in a mouse model of chronic gut inflammation.
Data obtained in the current study demonstrate that chronic gut inflammation is associated with enhanced plasma levels of granulo/myelopoietic cytokines, increased production of granulocytes and myeloid cells, and infiltration of large numbers of neutrophils into the MLNs and colon. Our data confirm those of Monteiro et al. (70) and Nemoto et al. (6) who demonstrated a robust myelopoietic response induced by adoptive transfer of CD4+ T cells into lymphopenic mice. In addition, the current study extends their findings by identifying, for the first time, to our knowledge, three major populations of myeloid cells that infiltrate the colon, the MLNs, and the spleen of mice with chronic colitis. Our detailed analyses identified Mac-1+Ly6ChighGr-1low/neg cells as monocytes, Mac-1+Ly6CintGr-1+ cells as mature and immature neutrophils, and Mac-1+Ly6Clow/negGr-1low/neg (DN) as a heterogeneous mixture of DCs, macrophage-like cells, and eosinophils. In all three tissues analyzed, we found that myeloid cells made up a significant portion of infiltrating leukocytes in colitic mice. In the colon, the combined number of Mac-1+Gr-1+ (which were mostly neutrophils) and Mac-1+Gr-1− cells (which contained monocytes and DN cells) were approximately the same as the number of CD4+ T cells (Fig. 2). In the MLNs, myeloid cells slightly outnumbered T cells, and in the spleen, we recovered ∼10-fold more myeloid cells (primarily neutrophils) than T cells.
On the basis of the cytospin and differential morphological analysis, neutrophils isolated from the colon were mostly “segmented” or mature, whereas a substantial number of those in the spleen had a donut-shaped nucleus characteristic of immature or “band” neutrophils (Supplemental Fig. 3). Interestingly, analysis of the peripheral blood obtained from colitic mice showed that majority of circulating neutrophils had mature, segmented morphology (Fig. 1E). This suggests that myelopoiesis observed during chronic inflammation, in addition to stimulating release of mature myeloid cells, also promotes egress of hematopoietic progenitors from the BM with their subsequent accumulation in the spleen. Recently, Swirski et al. (71) showed that during ischemic myocardial injury the spleen can serve as a reservoir for undifferentiated monocytes that are capable of rapidly migrating to the injured site where they participate in wound healing. Thus, in the context of chronic inflammation, the spleen may function as a “depot” for accumulation and storage of myeloid cells and their precursors, presumably, for their subsequent utilization to fight infection or, as has been proposed for monocytes (71), in wound healing. Although we (72) and others (73) previously demonstrated in this T cell transfer model lack of spleen in recipient immunodeficient mice had no affect on the induction of colitis, in view of our new data, it may be worthwhile to examine how splenectomy in mice with active colitis affects disease progression.
Another novel finding obtained in the current study is that neutrophils isolated from the chronically inflamed colon have acquired APC phenotype and function, based on their enhanced expression of MHC-II and CD86 on mRNA and protein levels as well as their ability to induce T cell activation in an Ag- and MHC-II–dependent manner. Although it can be argued that a small number of contaminating DCs and/or monocytes/macrophages could account for the APC phenotype and function, our in-depth multicolor flow cytometric analyses confirmed that these cells did not contain CD11c+ (DCs) or F4/80+ (macrophages) cells (Fig. 3B). We also show that induction of T cell proliferation is contingent upon MHC-II expression because blocking Ab completely abrogated proliferation of T cells. Last, we demonstrate that proliferation of T cells does not occur when Ag binds nonspecifically to the surface of fixed cells but is dependent on the ability of live neutrophils to internalize, process, and present Ag on their surface in association with MHC-II. These data are in agreement with several other reports demonstrating that neutrophils isolated from patients with chronic inflammatory conditions may undergo “transdifferentiation” into APCs and acquire the ability to induce T cell activation in vitro (47–49). Liu et al. (74) reported that peripheral monocytes isolated from CD patients showed increased expression of costimulatory molecules. “Transdifferentiation” refers to a process whereby a non-stem cell with a specific function transforms into a different type of cell and has been used frequently to describe acquisition of Ag-presenting characteristics by neutrophils (48, 49). This term, however, may not accurately reflect this phenomenon, because these cells retain morphology of neutrophils.
The specific mechanisms by which neutrophils acquire their APC function within the colon have not been identified; however, it has been demonstrated that culturing of circulating neutrophils with GM-CSF, IFN-γ, and/or IL-3 induces the expression of both MHC-II and costimulatory molecules (50–52). In addition to triggering conversion into APC-like cells, GM-CSF and IFN-γ can also delay apoptosis and increase the lifespan of granulocytes (54–56, 75–77). Indeed, acquisition of APC-like properties by neutrophils appears to be dependent on inflammatory cytokine-driven upregulation of MHC-II/CD86 because cells obtained after an acute inflammatory response (i.e., 4–6 h peritoneal exudates cells) do not show substantial surface expression of MHC-II or CD86 (Fig. 3E).
We also show that these extravasated neutrophils highly express accessory molecules that are important in T cell/APC interactions. For example, interaction between LFA-1 on T cells with ICAM-1 on APCs has been shown to be critical for optimal T cell activation (78, 79), and expression of ICAM-1 by APCs is thought to be important for the proper functioning of the immunological synapse during T cell activation (61, 80). Thus, a high level of ICAM-1 on cLP neutrophils is consistent with their more potent T cell stimulatory ability. In support of this contention, although we demonstrate that both the spleen and cLP neutrophils are capable of triggering Ag-specific T cell proliferation, those isolated from the colon are more potent as APCs (on a cell-to-cell basis) (Fig. 4A).
In addition to promoting T cell proliferation, we report that coculturing of neutrophils with T cells increased the production of different chemokines known to be chemoattractive for T cells (IP-10 and RANTES), neutrophils (KC), and monocytes (MCP-1) (Fig. 5). These data suggest that neutrophils may play an important role in promoting the recruitment of monocytes and effector T cells into the inflamed gut. Data presented in the current study also demonstrate that neutrophils, but not T cells, are the primary producers of the key granulopoietic and proinflammatory cytokines, such as IL-6 and G-CSF (81–84).
The molecular determinants required by neutrophils to migrate from the blood to the different tissues during the development of chronic gut inflammation have not been well defined. Under physiological conditions, L-selectin is known to promote neutrophil recruitment into the inflammatory sites (85). In this study, we found that circulating neutrophils in colitic mice express high levels of L-selectin (Fig. 6). Analysis of integrin expression revealed that circulating neutrophils also express high levels of the β2 (e.g., LFA-1 and Mac-1) and β1 integrins but no lymphocyte Peyer’s patch adhesion molecules-1, VLA-4, or αEβ7, suggesting that other integrins of the β1 family may be important mediators for promoting recruitment of neutrophils from the blood into the lymphoid (MLNs) and nonlymphoid (gut) tissues. With respect to adhesion molecules, it is noteworthy that we observed the highest level of β1 integrin expression on the surface of circulating neutrophils (Fig. 6). Analysis also revealed some interesting tissue-specific differences in surface molecule expression. For example, neutrophils isolated from the spleen and the MLNs of colitic mice expressed significant amounts of α4 integrin on their surface, whereas those in the blood or colon did not. Although it may reflect organ-specific induction, which is necessary for cellular localization within the specific tissue, we think that expression of α4 on the surface of neutrophils is an indicator of their immature phenotype (D.V. Ostanin and E. Kurmaeva, unpublished observations). Haile et al. (86) suggested that loss of α4 expression correlates with maturation of CD11b+Gr-1dull/int cells.
Taken together, our data demonstrate that chronic colitis is associated with a dramatic myelopoiesis, which correlates with the infiltration of large numbers of neutrophils into the colon and the MLNs. These innate immune cells acquire the phenotype and function of APCs that may provide additional stimulatory cues to colonic effector T cells, thereby perpetuating chronic gut inflammation. Because neutrophils represent a significant portion of cells infiltrating the intestine of patients with IBD, strategies aimed at reducing their numbers and/or function may prove useful as a therapeutic strategy for treating patients with IBD. The selective removal of granulocytes and monocytes through apheresis is one of the available treatment options for IBD patients in Japan and Europe. However, it should be remembered that although selective removal of granulocytes (e.g., neutrophils and eosinophils) may be beneficial, depletion of circulating monocytes, which function to promote tissue healing (42, 46), may counteract these positive effects. Indeed, it was recently reported that leukapheresis to remove both monocytes and neutrophils (87) failed to demonstrate clinical efficacy in a large-scale placebo-controlled clinical trial (88). Nevertheless, a better understanding of the roles that the different myeloid cells play in immune function and their contribution to the pathogenesis of chronic gut inflammation may ultimately reveal new therapeutic strategies for the treatment of patients with IBD.
Footnotes
This work was supported by a grant from the National Institutes of Health (PO1-DK 43785, Project 1, and Cores B and C) (to M.B.G.). D.V.O. is supported by funding from the Center of Excellence for Arthritis and Rheumatology (Louisiana State University Health Sciences Center, Shreveport, LA).
The online version of this article contains supplemental material.
References
Disclosures
The authors have no financial conflicts of interest.