Abstract
NK T cells are an unusual subset of T lymphocytes. They express NK1.1 Ag, are CD1 restricted, and highly skewed toward Vβ8 for their TCR usage. They express the unique potential to produce large amounts of IL-4 and IFN-γ immediately upon TCR cross-linking. We previously showed in the thymus that the NK T subset requires IL-7 for its functional maturation. In this study, we analyzed whether IL-7 was capable of regulating the production of IL-4 and IFN-γ by the discrete NK T subset of CD4+ cells in the periphery. Two hours after injection of IL-7 into mice, or after a 4-h exposure to IL-7 in vitro, IL-4 production by CD4+ cells in response to anti-TCR-αβ is markedly increased. In contrast, IFN-γ production remains essentially unchanged. In β2-microglobulin- and CD1-deficient mice, which lack NK T cells, IL-7 treatment does not reestablish normal levels of IL-4 by CD4+ T cells. Moreover, we observe that in wild-type mice, the memory phenotype (CD62L−CD44+) CD4+ T cells responsible for IL-4 production are not only NK1.1+ cells, but also NK1.1− cells. This NK1.1−IL-4-producing subset shares three important characteristics with NK T cells: 1) Vβ8 skewing; 2) CD1 restriction as demonstrated by their absence in CD1-deficient mice and relative overexpression in MHC II null mice; 3) sensitivity to IL-7 in terms of IL-4 production. In conclusion, the present study provides evidence that CD4+MHC class I-like-dependent T cell populations include not only NK1.1+ cells, but also NK1.1− cells, and that these two subsets are biased toward IL-4 production by IL-7.
Under Ag stimulation, naive CD4+ T cells develop into effector Th cells of the 1 or 2 phenotype (1). Th1 cells produce IL-2, IFN-γ, and TNF-β, which are involved in cell-mediated inflammatory reactions. Th2 cells secrete mainly IL-4, IL-5, IL-6, IL-10, and IL-13, which mediate B cell activation, Ab production, and negative regulation of Th1 responses. This developmental pathway is influenced by several factors (2, 3): the Ag dose, the type of APCs, and/or the costimulatory pathways. However, the most effective inducer of CD4+ T cell differentiation appears to be the local cytokine environment. It is clear that the cytokines IL-12 and IFN-γ direct differentiation to a Th1 phenotype. In many Th2 responses, IL-4 itself plays an essential role in the induction of naive T cells to subsequently produce IL-4 and other Th2-specific cytokines in response to Ag in vitro (4, 5) and in vivo (4).
It is still unknown, however, which cell population provides the earliest source of IL-4 required for specific Th2 responses in vivo (3). With regard to the mainstream CD4+ cell compartment, initial IL-4 could be produced by two distinct populations: 1) naive T cells in response to a primary stimulation in the presence of IL-6 (6); 2) already differentiated T cells that act on nearby naive T cells.
Recently, a subset of CD4+ T cells that express the NK1.1 molecule (7, 8), termed NK T cells, has been shown to rapidly secrete large amounts of IL-4 in the spleen upon activation with anti-CD3 Abs injected i.v. (9). CD4+ NK1.1+ T cells express intermediate level of TCR-αβ, have a strongly biased repertoire skewed toward Vβ8 (7, 8, 10), and are selected and restricted by nonclassical MHC class I molecules (7, 8, 11, 12), principally CD1 (13, 14, 15). Thus, the number of TCR-αβint NK1.1+ T cells is greatly reduced in the thymus and spleen of β2-microglobulin-deficient (β2m−/−)4 mice (11), and it has been proposed that this deficiency explains their low production of IL-4 and IgE upon anti-CD3 and anti-IgD injection (9, 16). Due to their capacity to rapidly produce IL-4 upon activation, it has been speculated that CD4+ NK1.1+ T cells may induce Ag-specific Th2 responses in vivo by providing significant amounts of IL-4 at the onset of an immune response (3, 7, 8, 16). However, interestingly, NK1.1+ T cells appear to possess a dual functionality. Besides IL-4, they secrete significant amounts of IFN-γ via the activation of the TCR/CD3 complex (15, 17). Moreover, they may be induced to produce large amounts of IFN-γ (but not IL-4) upon NK1.1 receptor cross-linking (18) or by proinflammatory cytokines such as IL-12 (18, 19).
It is likely that IL-4 dominates over IFN-γ (or IL-12), suggesting that Th2 cell development is favored in the presence of both cytokines (3, 5, 20). However, quantitative differences in IL-4 production during the initial stimulus may be an important factor influencing Th cell development and its stabilization (5, 21). In some circumstances, administration of IL-12 induces a marked inhibition of IL-4-secreting cells and may be indispensable in Ag-induced Th1 differentiation (22). Altogether, these observations support the idea that factors increasing the early IL-4 source would play a key role in promoting Th2 polarization.
Recent evidence from our laboratory suggests that IL-4 production by thymic NK T cells depends on or is facilitated by IL-7 (23, 24). First, thymic NK T cells from IL-7-deficient mice require IL-7 for their functional maturation into IL-4-producing cells (23). Second, a short 2-h preincubation of wild-type mouse thymic NK T cells with IL-7 is sufficient to significantly increase both the expression of IL-4 mRNA and IL-4 production capacity, and IL-4 has no effect in the same experimental conditions (24). Finally, the maintenance of IL-4 production by thymic NK T cells in vitro requires the specific presence of IL-7 (23, 25). Collectively, these findings suggest that IL-7 is important at a late stage of NK T cell development, i.e., to reach a stage of full maturation. Thus, from the standpoint of designing new effective vaccines, it will be relevant to determine whether, in the periphery, IL-7 has the ability to direct NK T cells toward a Th1 or a Th2 profile. We thus analyzed the modulatory effects of IL-7 in vivo and in vitro on direct IL-4 and IFN-γ production by CD4+ splenocytes. We show that IL-7 contributes to efficiently increase initial production of IL-4 (but not that of IFN-γ) by the CD4+ splenic T cell compartment. Cell sorting experiments using wild-type, CD1-deficient mice, β2m-deficient mice, or I-Aβ-deficient mice revealed that IL-7-responder CD4+ splenic T cells responsible for IL-4 production include, as expected, the NK T subset, but also an NK1.1− subset selected by class I-related molecules. Thus, by amplifying the early IL-4 sources, notably nonconventional CD4+ T cells, IL-7 may play a key role in promoting Th2 polarization.
Materials and Methods
Mice
Three- to ten-week-old female mice were used in this study. Wild-type and mutant (β2m−/− or I-Aβ−/−) C57BL/6 mice and wild-type BALB/c mice were bred and maintained in our animal facilities under specific pathogen-free conditions. Mutant β2m−/− mice of the BALB/c background were kindly provided by J. C. Guery (INSERM U28; CHU Purpan, Toulouse, France). Mutant CD1−/− mice (C57BL/6 and mixed C57BL/6 × 129 backgrounds) have been described previously (14).
Treatment of mice with IL-7
Human rIL-7, produced in Escherichia coli and purified by Immunex (Seattle, WA), was generously supplied by Sanofi (Labège, France). The IL-7 had a specific biological activity of 8.8 × 106 U/mg, as measured by the proliferation of a murine pre-B cell line. The endotoxin levels were 7.5 U/mg of IL-7. To test the effect of IL-7 on the production of IL-4 and IFN-γ by CD4+ T cell subpopulations, mice received s.c. one single injection of 2 μg of IL-7 diluted in a pyrogen-free solution containing BSA (500 μg/ml; Life Technologies, Gaithersburg, MD). Control mice were injected with an identical volume of the BSA solution alone. The in vivo biological effect of IL-7 was controlled by measuring the increase in both the absolute number of hemopoietic cells and the relative number of pre-B cells in the spleen of treated mice (26). For this, a 4-day treatment (2 μg twice daily) of IL-7 was performed and the mice were sacrificed 12 h after the last injection.
Abs and FACS analysis
FITC anti-CD44 (clone 1 M7.8), PE anti-NK1.1 (clone PK136), PE anti-CD4, FITC anti-IgM, and PE anti-B220 were obtained from PharMingen (San Diego, CA). Biotinylated anti-CD62L (clone Mel-14) and FITC anti-CD3 (clone 145-2C11) were kindly provided by F. Lepault (CNRS URA 1461, Institut Necker, Paris, France) and by L. Chatenoud (INSERM U25, Institut Necker), respectively. Biotinylated anti-IL-7R (clone A7R34) was a kind gift from T. Sudo (Toray Industries, Kanagawa, Japan). Staining was performed as described previously (12). After incubation with the biotinylated Abs, cells were incubated with the appropriate FITC- and PE-labeled mAbs plus streptavidin-Tricolor (SAv-Tri; Caltag, So. San Francisco, CA). Control staining with irrelevant Abs was always performed in parallel. A FACScan flow cytometer (Becton Dickinson, Mountain View, CA) was used. A minimum of 1 × 104 events gated on viable cells was acquired with CellQuest software. Results were analyzed using Mac CellQuest software.
Cell preparation
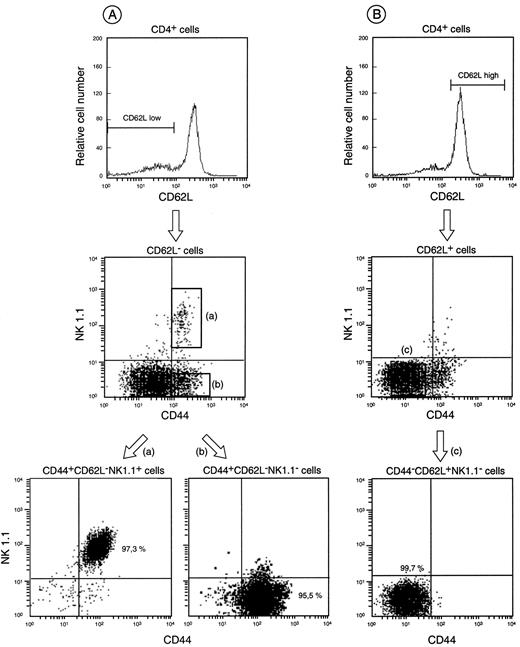
Spleen cell suspensions were prepared using a homogenizer and washed twice in PBS 1× supplemented with 5% FCS (Techgen, Les Ulis, France), and RBCs were lysed in a hemolyse buffer (NH4Cl, KHCO3, EDTA). Splenocytes were incubated for 45 min with anti-CD4-coated magnetic beads (Miltenyi Biotech, Bergisch-Gladbach, Germany) and positively sorted on a MACS positive selection column. In some experiments, enriched CD4+ splenocytes were stained with FITC anti-CD44, PE anti-NK1.1, and biotinylated anti-CD62L plus SAv-Tri. The CD44+CD62L−NK1.1+ (NK T cells), CD44+CD62L−NK1.1− (NK1.1− memory phenotype T cells), and CD44−CD62L+NK1.1− (naive phenotype T cells) populations were then sorted using a FACS Vantage cell sorter (Becton Dickinson). Purity of each enriched cell fraction was about 98% after reanalysis (see Fig. 3).
Purification procedure of CD4+ splenocytes based on the expression of CD44, CD62L, and NK1.1 markers. CD4+ splenocytes consist of a heterogeneous population that can be separated into three major subsets according to CD44, CD62L, and NK1.1 markers. A, CD44+CD62L−NK1.1+ and CD44+CD62L−NK1.1− subsets were obtained by MACS sorting of CD4+ splenocytes, staining with the appropriate mAbs (see Materials and Methods), and electronic sorting for CD44+NK1.1+ or CD44+NK1.1− cells (gates at the top and at the bottom of the flow cytometry dot plot) pregated on CD62L− cells. B, The CD44−CD62L+NK1.1− subset was obtained by MACS sorting of CD4+ splenocytes, staining with the appropriate mAbs (see Materials and Methods), and by electronic sorting for CD44−NK1.1− cells (gate of the flow cytometry dot plot) pregated on CD62L+ cells. Degree of purity of the sorted populations is indicated as percentage in each relevant gate. One representative experiment of 12 is shown.
Purification procedure of CD4+ splenocytes based on the expression of CD44, CD62L, and NK1.1 markers. CD4+ splenocytes consist of a heterogeneous population that can be separated into three major subsets according to CD44, CD62L, and NK1.1 markers. A, CD44+CD62L−NK1.1+ and CD44+CD62L−NK1.1− subsets were obtained by MACS sorting of CD4+ splenocytes, staining with the appropriate mAbs (see Materials and Methods), and electronic sorting for CD44+NK1.1+ or CD44+NK1.1− cells (gates at the top and at the bottom of the flow cytometry dot plot) pregated on CD62L− cells. B, The CD44−CD62L+NK1.1− subset was obtained by MACS sorting of CD4+ splenocytes, staining with the appropriate mAbs (see Materials and Methods), and by electronic sorting for CD44−NK1.1− cells (gate of the flow cytometry dot plot) pregated on CD62L+ cells. Degree of purity of the sorted populations is indicated as percentage in each relevant gate. One representative experiment of 12 is shown.
In vitro cultures and cytokine production
To assess cytokine production, cells in RPMI 1640 Glutamax culture medium (Life Technologies) supplemented with 10% FCS (Techgen), 0.05 mM 2-ME, penicillin (100 IU/ml), and streptomycin (100 μg/ml) were plated in triplicate (2.5 × 104 to 1 × 105/well; 200 μl final volume) in 96-well round-bottom microplates (Nunc, Roskilde, Denmark) coated with 10 μg/ml anti-TCR-αβ (H57-597). The supernatants were harvested 48 h later and stored at −80°C until IL-4 and IFN-γ assays. In some experiments, enriched CD4+ cells (4 × 105/well), NK1.1+ T cells (2 × 105/well), NK1.1− memory phenotype (2 × 105/well), or naive phenotype T cells (4 × 105/well) in 24-well plastic plates (0.5 ml final volume) were pretreated for 4 h with murine rIL-2 (50 U/ml; Genzyme, Cambridge, MA), murine rIL-4 (25 ng/ml; R&D Systems, Abingdon, U.K.), human rIL-7 (35 ng/ml), or culture medium alone. Cells were then extensively washed, and cultured with anti-TCR-αβ for 48 h in 96-well round-bottom microplates, as indicated above. Alternatively, naive phenotype T cells were cultured in 24-well plastic plates (2 × 105/well; 0.5 ml final volume) for 4 days in the presence of immobilized anti-TCR-αβ mAb and IL-2 (50 U/ml), with or without IL-4 (50 ng/ml). The cells were recovered and further incubated (4 × 105 cells/well) for 4 h in the presence or the absence of IL-7 (35 ng/ml). After extensive washing, the cells were rechallenged (1 × 105 cells/well) with immobilized anti-TCR-αβ mAb for 48 h in 96-well round-bottom microplates, as described above.
Cytokine assays
IL-4 content in supernatants of splenocyte cultures was quantitated in a bioassay using the IL-4-dependent CT.4S cell line (27), or with a two-site sandwich ELISA method (24) using 11B11 mAb for capture and biotinylated BVD6 mAb for detection. Responses from serial dilutions of the supernatants were compared with those elicited by known amounts of murine rIL-4 (R&D Systems). IFN-γ was measured by using a two-site sandwich ELISA (24): AN18 mAb was used for capture, and biotinylated R46A2 mAb was used for detection. IFN-γ and IL-4 concentrations are expressed in ng/ml, and were derived from calibration curves established with serial dilutions of mouse recombinant standards (R&D Systems) in each assay.
Statistical analysis
Differences in cytokine production were analyzed using Student’s t test.
Results
IL-7 up-regulates IL-4 production by splenic CD4+ T cells
This study was initiated to evaluate the role that IL-7 plays in influencing the primary T cell developmental pathway. To address this question, the effects of IL-7 on the production of IL-4 and IFN-γ by several spleen subpopulations of CD4+ T cells were analyzed using a simple in vitro system of primary stimulation with immobilized mAb to TCR-αβ. First, IL-4 and IFN-γ production by total splenic CD4+ T cells was determined after a 4-h exposure in vitro to IL-7. Splenic CD4+ T cells were isolated using anti-CD4 mAb-coated paramagnetic beads and the MACS device. As illustrated in Fig. 1,A, CD4+ splenocytes exposed to medium alone secreted significant IL-4 and IFN-γ upon TCR cross-linking. IL-7 treatment of splenic CD4+ cells in vitro resulted in a significant increase in IL-4 secretion, but had no effect on IFN-γ secretion. In contrast, both IL-2 and IL-4 failed to increase IL-4 secretion under the same experimental conditions. Because the proliferation rates were superimposable for IL-2, IL-4, and IL-7, we assume that the effect of IL-7 was not simply related to increased proliferation (see legend to Fig. 1).
In vitro and in vivo IL-7 treatment enhances IL-4 production, but not IFN-γ production by CD4+ splenocytes. CD4+ splenocytes were cultured (1 × 105 cells/well) with immobilized anti-TCR-αβ mAb (10 μg/ml, clone H57-597). Supernatants were harvested 48 h later and assayed for IL-4 and IFN-γ production. A, Purified CD4+ splenocytes from 4-wk-old C57BL/6 mice preincubated for 4 h with medium alone or with IL-2 (50 U/ml), IL-4 (50 ng/ml), or IL-7 (35 ng/ml). No significant difference was observed between proliferation rates (mean cpm ± SEM) of cells preincubated in the presence of IL-7 (302,232 cpm ± 20,590) as compared with those preincubated in the presence of IL-4 (320,933 cpm ± 9,705), or in the presence of IL-2 (328,459 cpm ± 20,214). B, Purified CD4+ splenocytes from 3- to 5-wk-old C57BL/6 mice sacrificed 2 h after treatment with IL-7 (2 μg) or BSA (excipient). The data are expressed as mean ± SEM from four experiments performed in triplicate.
In vitro and in vivo IL-7 treatment enhances IL-4 production, but not IFN-γ production by CD4+ splenocytes. CD4+ splenocytes were cultured (1 × 105 cells/well) with immobilized anti-TCR-αβ mAb (10 μg/ml, clone H57-597). Supernatants were harvested 48 h later and assayed for IL-4 and IFN-γ production. A, Purified CD4+ splenocytes from 4-wk-old C57BL/6 mice preincubated for 4 h with medium alone or with IL-2 (50 U/ml), IL-4 (50 ng/ml), or IL-7 (35 ng/ml). No significant difference was observed between proliferation rates (mean cpm ± SEM) of cells preincubated in the presence of IL-7 (302,232 cpm ± 20,590) as compared with those preincubated in the presence of IL-4 (320,933 cpm ± 9,705), or in the presence of IL-2 (328,459 cpm ± 20,214). B, Purified CD4+ splenocytes from 3- to 5-wk-old C57BL/6 mice sacrificed 2 h after treatment with IL-7 (2 μg) or BSA (excipient). The data are expressed as mean ± SEM from four experiments performed in triplicate.
Importantly, the ability of IL-7 to up-regulate IL-4 production by the splenic CD4+ T subset was confirmed in vivo in mice receiving a single 2 μg dose of the cytokine 2 h before analysis (Fig. 1 B). In these mice, IL-4 secretion was increased 2.5- to 6-fold, as compared with control mice treated with vehicle alone. In contrast, IFN-γ production remained essentially unchanged. Taken together, these results indicate that a short-term treatment of mice with IL-7 increases the IL-4/IFN-γ ratio in the spleen.
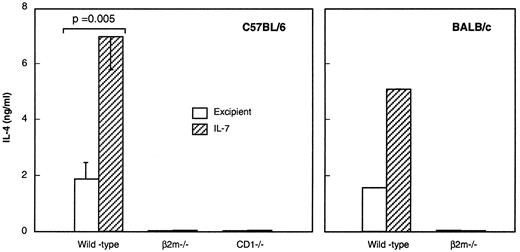
Class I-like/CD1-dependent selection is required for IL-7-driven IL-4 up-regulation in CD4+ T cells
Among potential IL-4-producing cells in the spleen, NK T cells are of particular interest because of their sensitivity to IL-7 in the thymus (12, 24, 25). To determine whether NK T cells are the target of IL-7 in our experimental system, we first analyzed the effect of IL-7 in β2m- and CD1-deficient mice, known to be defective in their selection of NK T cells (11, 12, 13, 14, 15). Splenic CD4+ T cells from C57BL/6 and BALB/c β2m- or CD1-deficient mice were found to be severely impaired in their capacity to rapidly release IL-4. Importantly, the in vivo administration of IL-7 did not normalize IL-4 production by CD4+ splenocytes from these mice (Fig. 2). To exclude the possibility that this effect was due to insufficient bioavailability of IL-7 or to insensitivity to IL-7 in the spleen, we tested a well-characterized surrogate marker, IL-7-induced B cell lymphopoiesis (26), for IL-7 activity in vivo. After a 4-day IL-7 treatment, we observed increased cellularity (data not shown) and frequency of the particular IgM− B220+ pre-B cell subset in all mice tested, including β2m- and CD1-deficient mice (Table I).
Enhancement of IL-4 production by CD4+ splenocytes in response to IL-7 involves β2m/CD1-dependent T cells. Four- to six-week-old wild-type, CD1- and β2m-deficient mice from the indicated strain were treated with IL-7 for 2 h and then sacrificed. Purified CD4+ splenocytes were cultured (1 × 105 cells/well) with immobilized anti-TCR-αβ mAb. Supernatants were harvested 48 h later and assayed for IL-4 production. The data are the mean ± SEM of two (β2m−/−, CD1−/−) to five (wild-type) experiments, except in BALB/c mice (one experiment).
Enhancement of IL-4 production by CD4+ splenocytes in response to IL-7 involves β2m/CD1-dependent T cells. Four- to six-week-old wild-type, CD1- and β2m-deficient mice from the indicated strain were treated with IL-7 for 2 h and then sacrificed. Purified CD4+ splenocytes were cultured (1 × 105 cells/well) with immobilized anti-TCR-αβ mAb. Supernatants were harvested 48 h later and assayed for IL-4 production. The data are the mean ± SEM of two (β2m−/−, CD1−/−) to five (wild-type) experiments, except in BALB/c mice (one experiment).
Biological effect of in vivo IL-7 treatment: an increase in splenic pre-B cell frequencya
| . | % IgM−B220+ Cells . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | C57BL/6 . | . | . | BALB/c . | . | ||||
| . | Wild type . | β2m−/− . | CD1−/− . | Wild type . | β2m−/− . | ||||
| Excipient | 3.7b | 3.4 | 3.4 | 6.4 | 6.4 | ||||
| IL-7 | 16.7 | 14.9 | 30.0 | 25.4 | 17.0 | ||||
| . | % IgM−B220+ Cells . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | C57BL/6 . | . | . | BALB/c . | . | ||||
| . | Wild type . | β2m−/− . | CD1−/− . | Wild type . | β2m−/− . | ||||
| Excipient | 3.7b | 3.4 | 3.4 | 6.4 | 6.4 | ||||
| IL-7 | 16.7 | 14.9 | 30.0 | 25.4 | 17.0 | ||||
Three- to 5-wk-old wild-type, β2m- and CD1-deficient mice from the indicated strain were treated for 4 days with IL-7 (2 μg twice daily). On the fifth day, total fresh splenocytes were removed and stained for flow cytometry with the appropriate mAb.
The pre-B cell frequency is expressed as the percentage of IgM−B220+ cells. The data are from one representative experiment of two (β2m+/+) to three (β2m−/−) except for BALB/c and CD1−/− mice (one experiment).
Second, we studied the effect of IL-7 treatment on the burst of IL-4 production induced by a single i.v. injection of CD3 Abs. In this model, the IL-4 burst is mostly attributed to NK T cells because of its relative absence in β2m-deficient mice (9). IL-7 treatment significantly increased the anti-CD3-triggered IL-4 production by splenic T cells from wild-type mice, while it did not reverse the deficient IL-4 production in β2m-deficient mice (Ref. 24 and data not shown). As a whole, these results point to the MHC class I-like-dependent CD4+ NK T cell population as the principal target of IL-7 in our system.
CD4+ NK1.1+ T cells are a major but not the only target of IL-7 for IL-4 production
To directly test whether CD4+ NK T cells were the exclusive target of IL-7, we purified this minor cell subset comprising less than 5% of total CD4+ splenic T cells. We took advantage of its particular phenotype showing an intermediate TCR-αβ surface density, the presence of the NK1.1 and the CD44 markers, and the absence of the CD62L marker (28). Electronic cell sorting of MACS-separated CD4+ splenic cells using biotinylated Mel-14 (anti-CD62L mAb), PE-PK136 (anti-NK1.1 mAb), and FITC anti-CD44 mAb led routinely to approximately 95–98% pure CD44+CD62L−NK1.1+TCR-αβint cells (see Fig. 3,A). The CD4+NK1.1− subset included both CD44+CD62L− and CD44−CD62L+ cells (see Fig. 3 for purification procedure). As shown in Tables II and III, highly purified CD4+ CD44+CD62L−NK1.1+ cells produced considerable amounts of IL-4 upon TCR cross-linking. However, purified CD4+ CD44+CD62L−NK1.1− cells also produced significant amounts of IL-4, although to a lesser degree than CD4+NK1.1+ cells. In contrast, CD4+ CD44−CD62L+NK1.1− cells, namely naive phenotype T cells (3, 29), produced virtually no IL-4 (<0.02 ng/ml) in the same culture conditions. Unlike CD4+ CD44+CD62L− cells, CD4+ CD44−CD62L+ cells acquired their potential to produce IL-4 only upon restimulation, and such a production depended on the presence of an initial IL-4 source during their priming (4, 5) (Fig. 5). Thus, the data strongly suggest that the IL-4-producing assay used in the present work reveals exclusively direct IL-4 production.
Conventional IL-4-producing CD4+ T cells respond to IL-7 by enhancing IL-4 production. Naive phenotype CD4+ T cells from 8-week-old β2m-deficient C57BL/6 mice were obtained after staining of purified CD4+ splenocytes and cell sorting by flow cytometry, as described in Fig. 3. Cells were cultured (2 × 105 cells/well) for 4 days in the presence of immobilized anti-TCR mAb and IL-2 (50 U/ml) with or without IL-4 (50 ng/ml). The recovered cells were then incubated (4 × 105 cells/well) for 4 h in the presence or the absence of IL-7 (35 ng/ml). After extensive washing, the cells were cultured (1 × 105 cells/well) with immobilized anti-TCR mAb. Supernatants were harvested 48 h later and assayed for IL-4 and IFN-γ production. The data are expressed as nanograms per milliliter and represent one typical experiment performed in triplicate.
Conventional IL-4-producing CD4+ T cells respond to IL-7 by enhancing IL-4 production. Naive phenotype CD4+ T cells from 8-week-old β2m-deficient C57BL/6 mice were obtained after staining of purified CD4+ splenocytes and cell sorting by flow cytometry, as described in Fig. 3. Cells were cultured (2 × 105 cells/well) for 4 days in the presence of immobilized anti-TCR mAb and IL-2 (50 U/ml) with or without IL-4 (50 ng/ml). The recovered cells were then incubated (4 × 105 cells/well) for 4 h in the presence or the absence of IL-7 (35 ng/ml). After extensive washing, the cells were cultured (1 × 105 cells/well) with immobilized anti-TCR mAb. Supernatants were harvested 48 h later and assayed for IL-4 and IFN-γ production. The data are expressed as nanograms per milliliter and represent one typical experiment performed in triplicate.
A substantial increase in IL-4 production was found in cultures of purified CD4+ CD44+CD62L−NK1.1+ and CD4+ CD44+CD62L−NK1.1− splenic T cells from mice injected with a single dose of IL-7 2 h before killing, as compared with untreated controls (Table II). These results were confirmed in vitro, indicating a direct effect of IL-7 on each of the NK1.1− and the NK1.1+ subsets sharing the CD44+CD62L− phenotype (Table III). Indeed, exposure of these subsets for 4 h to IL-7 before anti-TCR-αβ stimulation was sufficient to lead to a significant 2- to 3-fold increase in IL-4 production (Table III). In contrast, purified CD4+ CD44−CD62L+NK1.1− T cells produced low amounts of IL-4 (<0.02 ng/ml) upon TCR cross-linking following exposure to IL-7 in vivo as well as in vitro (Tables II and III). FACS analysis using an anti-IL-7R indicates that the lack of responsiveness to IL-7 was not due to lower IL-7R expression by CD44−CD62L+NK1.1− T cells, as compared with the two other CD4+ populations tested (data not shown). Finally, both the CD44+CD62L−NK1.1+ and the CD44+CD62L−NK1.1− subsets produced low amounts of IFN-γ in response to anti-TCR-αβ (less than 0.33 ng/ml per 2.5 × 104 cells), and IL-7 had no effect (less than 0.25 ng/ml per 2.5 × 104 cells).
In vivo IL-7 treatment enhances IL-4 production by both NK1.1− and NK1.1+ subsets of CD4+ CD44+CD62L− T splenocytesa
| Splenic CD4+ T Cells . | β2m+/+ (wild type) . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | Expt. 1 . | . | Expt. 2 . | . | β2m−/− . | . | |||
| . | Excipient . | IL-7 . | Excipient . | IL-7 . | Excipient . | IL-7 . | |||
| CD44+CD62L−NK1.1+ | 0.5b | 1.4 | 0.74 | 2.2 | — | — | |||
| CD44+CD62L−NK1.1− | 0.15 | 2.2 | 0.14 | 0.44 | 0.02 | 0.03 | |||
| CD44−CD62L+NK1.1− | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | |||
| Splenic CD4+ T Cells . | β2m+/+ (wild type) . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | Expt. 1 . | . | Expt. 2 . | . | β2m−/− . | . | |||
| . | Excipient . | IL-7 . | Excipient . | IL-7 . | Excipient . | IL-7 . | |||
| CD44+CD62L−NK1.1+ | 0.5b | 1.4 | 0.74 | 2.2 | — | — | |||
| CD44+CD62L−NK1.1− | 0.15 | 2.2 | 0.14 | 0.44 | 0.02 | 0.03 | |||
| CD44−CD62L+NK1.1− | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | |||
Four- to six-week-old wild-type or β2m-deficient C57BL/6 mice were injected once with IL-7 (2 μg) or BSA (excipient) and were sacrificed 2 h after treatment. The indicated subsets were purified as described in Fig. 3 and cultured (2.5 × 104 cells/well) with immobilized anti-TCR-αβ mAb.
Supernatants were harvested 48 h later and assayed for IL-4 production (ng/ml). The data are from two experiments of three for β2m+/+ mice and from one of two for β2m−/− mice.
In vitro IL-7 treatment enhances IL-4 production by both NK1.1− and NK1.1+ subsets of CD4+ CD44+CD62L− T splenocytesa
| Splenic CD4+ T Cells . | β2m+/+ (wild type) . | . | β2m−/− . | . | ||
|---|---|---|---|---|---|---|
| . | Medium . | IL-7 . | Medium . | IL-7 . | ||
| CD44+CD62L−NK1.1+ | 1.9b | 3.5 | — | — | ||
| CD44+CD62L−NK1.1− | 0.20 | 0.77 | 0.03 | 0.02 | ||
| CD44−CD62L+NK1.1− | <0.02 | <0.02 | <0.02 | <0.02 | ||
| Splenic CD4+ T Cells . | β2m+/+ (wild type) . | . | β2m−/− . | . | ||
|---|---|---|---|---|---|---|
| . | Medium . | IL-7 . | Medium . | IL-7 . | ||
| CD44+CD62L−NK1.1+ | 1.9b | 3.5 | — | — | ||
| CD44+CD62L−NK1.1− | 0.20 | 0.77 | 0.03 | 0.02 | ||
| CD44−CD62L+NK1.1− | <0.02 | <0.02 | <0.02 | <0.02 | ||
The indicated subsets (see legend of Fig. 3) were isolated from 6- to 8-wk-old wild-type or β2m-deficient untreated C57BL/6 mice. Cells were incubated in vitro with IL-7 (35 ng/ml) or medium alone for 4 h, and then cultured (2.5 × 104 cells/well) with immobilized anti-TCR-αβ mAb. Supernatants were harvested 48 h later and assayed for IL-4 production.
The data expressed as ng/ml represent one typical experiment of three.
IL-4-producing NK1.1− CD4+ T cells responsive to IL-7 are selected by class I-related molecules
To determine the origin of the IL-4-producing NK1.1− CD4+ T cells that respond to IL-7, we analyzed mice lacking CD1 molecules, MHC class I molecules (i.e., β2m−/− mice), or MHC class II molecules (i.e., I-Aβ−/− mice). In contrast to β2m-deficient mice that displayed a 2-fold reduction in the size of the CD4+ CD44+CD62L−NK1.1− T compartment (about 10–15% of the splenic CD4+ compartment instead of 25–30% in wild-type mice), I-Aβ−/− mice contained a large majority of these cells (approximately 50–60% of the splenic CD4+ compartment), suggesting the β2m dependence of this subset for its selection. Furthermore, CD4+ CD44+NK1.1− T cells, like CD4+ CD44+NK1.1+ T cells, are highly skewed toward Vβ8 TCR usage in wild-type and I-Aβ−/− mice when compared with their naive counterpart, CD4+ CD44−NK1.1− cells (Table IV). Vβ8 overexpression was not observed in the CD4+ CD44+NK1.1− cell compartment from β2m−/− mice, confirming the existence of a class I-dependent NK1.1− T cell population expressing a TCR repertoire biased to Vβ8. Finally, the absence of Vβ8 bias in CD4+ CD44+NK1.1− T cells from CD1−/− mice provides direct evidence for the CD1 dependence of this population (Table IV).
Frequency of cells expressing Vβ8 among TCR-αβ+ CD4+ splenocytes according to CD44 and NK1.1 expression in wild-type, β2m-deficient, CD1-deficient, and I-Aβ-deficient micea
| . | Frequency of Vβ8 Cells . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | Wild type . | I-Aβ−/− . | β2m−/− . | CD1−/− . | |||
| CD44−NK1.1− | 21.2 ± 0.6† | 22.6 ± 1.9‡ | 19.4 ± 0.6 | 22.2 ± 0.1 | |||
| CD44+NK1.1− | 33.0 ± 3.0 | 46.8 ± 3.4 | 18.0 ± 0.5 | 21.5 ± 0.2 | |||
| CD44+NK1.1+ | 66.0 ± 2.0§ | 61.9 ± 3.6§ | |||||
| . | Frequency of Vβ8 Cells . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | Wild type . | I-Aβ−/− . | β2m−/− . | CD1−/− . | |||
| CD44−NK1.1− | 21.2 ± 0.6† | 22.6 ± 1.9‡ | 19.4 ± 0.6 | 22.2 ± 0.1 | |||
| CD44+NK1.1− | 33.0 ± 3.0 | 46.8 ± 3.4 | 18.0 ± 0.5 | 21.5 ± 0.2 | |||
| CD44+NK1.1+ | 66.0 ± 2.0§ | 61.9 ± 3.6§ | |||||
CD4-enriched splenocytes from 4- to 11-wk-old mice of the indicated strain were stained as described in Materials and Methods and analyzed on gated CD4+ cells for the expression of TCR-αβ or Vβ8 according to CD44 and NK1.1 expression. Frequency of Vβ8+ cells was calculated as % (Vβ8+ cells in the indicated subset) / % (TCR-αβ+ cells on the same subset). The data are the arithmetic mean ± SEM of three experiments for wild-type mice, six for I-Aβ−/− mice, and two for both β2m−/− and CD1−/− mice. Statistical significance at † p<0.05, ‡ p<0.005, and § p<0.001 when Vβ8 frequency among CD44+NK1.1− cells is compared with that of CD44−NK1.1− and CD44+NK1.1+ cells for each strain of mice indicated.
IL-4 production by CD4+ CD44+CD62L−NK1.1− T cells was not detected in β2m-deficient mice, confirming that the development of NK1.1− IL-4-producing T cells in unmanipulated mice is β2m dependent (Tables II and III). It must be emphasized that this defect could not be reversed by IL-7 in vivo and in vitro. As indicated in Fig. 4, splenic CD4+ CD44+CD62L−NK1.1− T cells from I-Aβ−/− mice treated with IL-7 produced more IL-4 upon TCR cross-linking than their counterparts from untreated mice. This finding definitively confirms the direct activity of IL-7 on the NK1.1− MHC class I-like-dependent CD4+ T cell population. Finally, as in wild-type mice (Table II), no modification in IFN-γ production by CD4+ CD44+CD62L−NK1.1− T cells was evidenced in response to IL-7 treatment of I-Aβ−/− mice (Fig. 4).
IL-4 production by CD4+ NK1.1− splenocytes from class II-deficient mice is increased by IL-7. Four-week-old class II-deficient C57BL/6 mice were injected once with IL-7 (2 μg) or BSA (excipient) and were sacrificed 2 h after treatment. CD4+ NK1.1− splenocytes were purified as described in Fig. 3 and cultured (5 × 104 cells/well) with immobilized anti-TCR-αβ mAb. Supernatants were harvested 48 h later and assayed for IL-4 and IFN-γ production. The data represent one typical experiment of two.
IL-4 production by CD4+ NK1.1− splenocytes from class II-deficient mice is increased by IL-7. Four-week-old class II-deficient C57BL/6 mice were injected once with IL-7 (2 μg) or BSA (excipient) and were sacrificed 2 h after treatment. CD4+ NK1.1− splenocytes were purified as described in Fig. 3 and cultured (5 × 104 cells/well) with immobilized anti-TCR-αβ mAb. Supernatants were harvested 48 h later and assayed for IL-4 and IFN-γ production. The data represent one typical experiment of two.
Increased IL-4 production by conventional CD4+ Th2 cells in response to IL-7
To determine the effect of IL-7 on conventional IL-4-producing cells, we took advantage of the fact that the presence of IL-4 during in vitro priming determines the IL-4-producing potential of CD4+ naive T cells upon restimulation (4, 5). In our hands, memory CD4+ T cells from β2m-deficient mice produced IL-4 upon rechallenge provided they were primed in the presence of IL-4 (Fig. 5). Pretreatment with IL-7 led to a dramatic (5-fold) increase in IL-4 production, as compared with pretreatment with medium alone. This suggests that IL-7 is able to increase IL-4 production in conventional memory CD4+ T cells provided their IL-4-producing potential was acquired during their development.
Discussion
IL-7 was originally identified as a growth factor for B cell progenitors (30, 31), but is now considered as a pleiotropic cytokine with proliferation and differentiation effects on both B and T lymphocytes in vitro (32, 33) and in vivo (26, 34). The marked B and T lymphopenia and reduced functional competence of T cells in IL-7-deficient mice (35) are consistent with a central role for IL-7 in the maturation processes of lymphoid cell lineages.
In the context of T lymphocyte-dependent immune responses (36, 37, 38, 39, 40, 41), IL-7 supports proliferation of mature CD8+ T cells (32, 42, 43), and enhances the cytolytic activity of antiviral (44, 45) and antitumoral CD8+ CTL (46) in humans as well as in mice. IL-7 also supports proliferation of CD4+ T cells (32, 47), but the role of this cytokine on CD4+ Th cell differentiation pathways is not well understood (38, 40, 48).
We show herein that IL-7 has the ability to increase rapidly and efficiently the IL-4-producing capacity of splenic CD4+ T cells. A 4-h preincubation with IL-7 renders CD4+ spleen cells capable of producing significantly more IL-4 upon stimulation by TCR cross-linking than untreated CD4+ cells. Likewise, CD4+ spleen cells can be sensitized in vivo by IL-7. It is noteworthy that IL-7 treatment both in vivo and in vitro does not modify the IFN-γ production by CD4+ spleen cells. Moreover, 2 h after the s.c. administration of IL-7, both the production of IL-4 in response to TCR in vitro and the IL-4 burst, which follows a challenge with anti-CD3 Abs in vivo, were improved. To our knowledge, these results represent the first successful use of a cytokine in vivo other than IL-4 for the up-regulation of direct IL-4 secretion by CD4+ T cells.
Three lines of evidence favor the involvement of CD4+ NK T cells as a target for IL-7: 1) The action of IL-7 on IL-4 production by CD4+ spleen cells (stimulated either by anti-CD3 in vivo or anti-TCR in vitro) cannot be demonstrated in β2m- and in CD1-deficient mice that lack NK T cells; 2) freshly sorted CD4+ NK1.1+ T cells from mice treated for 2 h with IL-7 produce more IL-4 upon TCR cross-linking than CD4+ NK1.1+ T cells from mice treated with excipient alone; 3) similarly, a single in vitro preincubation of CD4+ NK1.1+ T cells from unmanipulated mice with IL-7 induces an increase in IL-4 production as compared with control cells.
Analysis of sorted populations indicates that the CD4+ T cells responsible for IL-4 production and its increase in response to IL-7 are not only NK1.1+ T cells, but also NK1.1− T cells that share both a memory phenotype (CD44+CD62L−) and Vβ8 skewing with NK1.1+ T cells. NK1.1−CD44+ CD4+ T cells from class II-deficient mice exhibited higher IL-4 production and Vβ8 frequency when compared with normal mice. Conversely, IL-4-secreting CD4+ T cells were not detected in β2m- and CD1-deficient mice for which no Vβ8 skewing could be demonstrated in the NK1.1−CD44+ CD4+ T population. These results indicate that a large proportion of NK1.1− IL-4-producing cells are CD1 restricted and include NK T cells that have lost NK1.1 Ag expression. In accordance with our findings, Cheng and Paul (49) have recently identified a subset of NK1.1− CD4+ T cells that exhibit a Vβ8 skewing and that produce high levels of IL-4 upon stimulation in vitro. However, in class II-deficient mice as in wild-type mice, the frequency of cells expressing Vβ8 is lower in the CD44+NK1.1− CD4+ population, when compared with the NK1.1+ CD4+ population (see Table IV). One possible explanation for this finding might be the existence of CD1-selected NK1.1− IL-4-producing cells that are distinct from NK T cells. Our demonstration of an enhancing effect of IL-7 on IL-4 production by the CD4+ CD44+CD62L−NK1.1− splenic compartment in class II-deficient mice, but not in β2m-deficient mice, reinforces the view that IL-7 acts not only on NK1.1+ cells, but also on NK1.1− cells that are specific for class I-related molecules.
Along with CD1-dependent NK.1.1− and NK1.1+ T cells, conventional (class II-restricted) CD4+-differentiated Th2 cells may also be a source of early IL-4. From our experiments, we cannot exclude that IL-4 production did not result in part from MHC class II-restricted memory (CD44+CD62L−) CD4+ T cells previously primed by environmental or self Ags in vivo. This is in accordance with the lower frequency of cells expressing Vβ8 in the wild-type CD4+ CD44+NK1.1− population, as compared with that of the CD4+ CD44+NK1.1− population from I-Aβ−/− mice (see Table IV). Due to the difficulty in discriminating between conventional and nonconventional NK1.1− cells among CD4+ memory phenotype T cells in normal mice ex vivo, we addressed the question of whether IL-7 regulates IL-4 production by mainstream CD4+-differentiated Th2 lymphocytes using in vitro generated cells. The finding that a 4-h IL-7 treatment in vitro is sufficient to drastically increase IL-4 production by these cells leads us to propose that IL-7 plays an important role in Th2 differentiation.
Another important question is the mechanism for the putative IL-7 action on NK1.1+ and NK1.1− IL-4-producing T cells. We previously showed that IL-7 was particularly effective in promoting NK T cell expansion in vitro (12). Herein, the action of IL-7 cannot be explained only by an increase in the generation of IL-4-producing cells since other cytokines such as IL-2 and IL-4, known to induce the proliferation of T cells (see legend to Fig. 1), failed to increase IL-4 production by CD4+ splenocytes. This suggests a key role for the IL-7R α-chain since the γ-chain is common to IL-2, IL-4, and IL-7 receptors. Such a conclusion is consistent with a recent report by Boesteanu et al. (50) showing distinct roles for signals relayed through the common cytokine receptor γ-chain and IL-7R α-chain in NK T cell development. Although IL-7 stimulation alone does not result in detectable IL-4 secretion (data not shown), it significantly increases IL-4 mRNA levels in NK T subsets (24). Whether IL-7 has the ability to stabilize IL-4 mRNA needs further investigation.
A possible role of NK T cells in the regulation of both Th1 and Th2 immune responses has been recently proposed (16, 51, 52, 53, 54). Cytokine production by these cells, either of the Th1 or Th2 type, is partially dependent on the cytokine profile present in their microenvironment. IL-12 induces IFN-γ production by NK T cells (18, 19), suggesting that IL-12 is important in driving NK T-dependent immune reactions toward a Th1-type response (53, 54). In contrast, IL-7 drives NK T cells toward the Th2-type phenotype. However, the possibility that the action of IL-7 on CD1-dependent CD4+ T cells is sufficient alone to drive Th2 differentiation is speculative since it has been shown that mice selectively deficient in this population are capable of normal Th2 responses (55, 56, 57). However, the fact that the action of IL-7 is not confined to class I-like-dependent CD4+ T populations, and may also involve conventional CD4+ Th2 populations reinforces the putative role of IL-7 as an important factor for Th2 differentiation.
In conclusion, the present study provides evidence that a large spectrum of CD4+ splenic cells, including NK1.1+ and NK1.1− MHC class I-like-dependent CD4+ T cells, as well as conventional NK1.1− MHC class II-restricted CD4+ T cells, are biased toward IL-4 production by IL-7. This observation supports the idea that IL-7 favors a Th2-type profile, by potentiating early IL-4 sources, and facilitating the ongoing IL-4 secretion by primed CD4+ T cells. Given the above, IL-7 could be a useful tool in developing future immune deviation strategies.
Acknowledgements
We thank L. Chatenoud, M. Dy, F. Lepault, and M. Throsby for critically reading the manuscript. We thank C. Garcia for excellent assistance with the FACS Vantage cell sorter, I. Cisse for managing the specific pathogen-free mouse colonies, D. Broneer for reviewing the manuscript, and M. Netter and M. Lillié for the art work. We are specially indebted to Sanofi Compagny (Labège, France) and to T. Sudo (Toray Industries, Kanagawa, Japan) for providing human rIL-7 and anti-IL-7R (clone A7R34), respectively.
Footnotes
This work was supported by institute funds from the INSERM and the Ligue Nationale contre le Cancer (Axe Immunology, 1998).
Abbreviation used in this paper: β2m, β2-microglobulin.