Abstract
IL-27 is a novel IL-6/IL-12 family cytokine playing an important role in the early regulation of Th1 responses. We have recently demonstrated that IL-27 has potent antitumor activity, which is mainly mediated through CD8+ T cells, against highly immunogenic murine colon carcinoma. In this study, we further evaluated the antitumor and antiangiogenic activities of IL-27, using poorly immunogenic murine melanoma B16F10 tumors, which were engineered to overexpress single-chain IL-27 (B16F10 + IL-27). B16F10 + IL-27 cells exerted antitumor activity against not only s.c. tumor but also experimental pulmonary metastasis. Similar antitumor and antimetastatic activities of IL-27 were also observed in IFN-γ knockout mice. In NOD-SCID mice, these activities were decreased, but were still fairly well-retained, suggesting that different mechanisms other than the immune response are also involved in the exertion of these activities. Immunohistochemical analyses with Abs against vascular endothelial growth factor and CD31 revealed that B16F10 + IL-27 cells markedly suppressed tumor-induced neovascularization in lung metastases. Moreover, B16F10 + IL-27 cells clearly inhibited angiogenesis by dorsal air sac method, and IL-27 exhibited dose-dependent inhibition of angiogenesis on chick embryo chorioallantoic membrane. IL-27 was revealed to directly act on HUVECs and induce production of the antiangiogenic chemokines, IFN-γ-inducible protein (IP-10) and monokine induced by IFN-γ. Finally, augmented mRNA expression of IP-10 and monokine induced by IFN-γ was detected at the s.c. B16F10 + IL-27 tumor site, and antitumor activity of IL-27 was partially inhibited by the administration of anti-IP-10. These results suggest that IL-27 possesses potent antiangiogenic activity, which plays an important role in its antitumor and antimetastatic activities.
Recently, a new member of the IL-6/IL-12 family of cytokines, IL-27, was identified (1, 2, 3, 4, 5). IL-27 is a heterodimeric cytokine that consists of an EBV-transformed gene 3 (EBI3), an IL-12p40-related protein, and p28, a newly discovered IL-12p35-related polypeptide. The former subunit was originally described as a factor secreted by EBI3, while the latter was identified through its homology to the IL-6/IL-12 cytokine family. IL-27 is produced early by activated APCs and induces a rapid clonal expansion of naive but not memory CD4+ T cells and synergizes with IL-12 to trigger IFN-γ production in naive CD4+ T cells (1). IL-27R consists of WSX-1 (also known as T cell cytokine receptor) and gp130 subunits (6). WSX-1 is expressed primarily in lymphoid tissues and its expression is highest in naive T and NK cells. Coexpression of both IL-27R subunits can be detected in many immune cell types. Binding of IL-27 to IL-27R induces phosphorylation of STAT1, 2, 3, 4, and 5 (7, 8, 9, 10), and augments T-bet expression, which is mainly mediated through STAT1 activation (10). T-bet then stimulates the development of Th1-type immune responses by transactivating the IFN-γ gene and up-regulating IL-12Rβ2 expression.
Angiogenesis is an essential process for tumors and metastases to grow in vivo and is the result of a complex balance between angiogenic and antiangiogenic factors (11, 12). Vascular endothelial growth factor (VEGF)3 is the most potent angiogenic cytokine (13). The expression of VEGF is stimulated by hypoxia, which is persistent and frequently found in tumor cells (14). Inhibition of the development of new blood vessels in tumors and metastases is effective in suppressing growth of these tumors. Endogenous angiogenic inhibitors such as angiostatin (15) and endostatin (16), CXC chemokines (17) such as platelet factor 4 (CXCL4) (18), IFN-γ-inducing protein (IP-10) (CXCL10) (19, 20), monokine induced by IFN-γ (MIG) (CXCL9) (21), and several cytokines such as IL-4 (22), IL-12 (23, 24), and IL-18 (24, 25) were reported to inhibit tumor growth and metastasis through their antiangiogenic effects. Tumor angiogenesis is a complex multistep process as follows: 1) production of angiogenic factors by tumor cells; 2) activation of the biological functions of vascular endothelial cells by the angiogenic factors; 3) degradation of the basement membrane of endothelial cells by enzymes; 4) migration and proliferation of endothelial cells; and 5) tube formation of endothelial cells (11, 12). Effective inhibition of any single step in the angiogenic cascade could lead to suppression of angiogenesis and therefore tumor growth.
We and other researchers have recently demonstrated that IL-27 has a potent ability to induce tumor-specific antitumor and protective immunity using colon carcinoma colon 26 (C26) (26, 27) and TBJ neuroblastoma (28). However, the process of the antiangiogenic activity of IL-27 remains to be elucidated. In the present study, we evaluated whether or not IL-27 possesses antiangiogenic activity and whether this ability plays an important role in antitumor and antimetastatic activities.
Materials and Methods
Mice
C57BL/6 and ICR mice were purchased from SLC Japan and Charles River Laboratories, respectively. C57BL/6 IFN-γ knockout (KO) (29) and NOD NOD-scid/scid mice (30) were maintained at Tokyo Medical University. All mice were used in accordance with the institutional guidelines of Tokyo Medical University and Tokyo Metropolitan Institute of Medical Science. This study was reviewed and approved by institutional review committees of both Tokyo Medical University and Tokyo Metropolitan Institute of Medical Science.
Reagents
Anti-STAT1 and anti-STAT3 were from Santa Cruz Biotechnology. Anti-phosphotyrosine (pY)-STAT1 and anti-pY-STAT3 were from Cell Signaling Technology. Human rIFN-γ was provided by Shionogi and Company. Anti-FLAG (M2) and biotinylated anti-IL-27 p28 were from Sigma-Aldrich and R&D Systems, respectively.
Cells
B16F10 malignant melanoma and HEK293T human kidney cells were cultured in RPMI 1640 and DMEM medium, respectively, supplemented with 10% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin. HUVEC (Sanko Junyaku) were cultured in EBM-2 medium (Sanko Junyaku) containing 2% FBS, human epidermal growth factor, hydrocortisone, VEGF, human fibroblast growth factor-B, R3-IGF-1, ascorbic acid, heparin, gentamicin, and amphotericin B.
Preparation of B16F10 transfectants
Mouse IL-12 p40 and IL-27 EBI3 and p28 cDNAs were isolated by RT-PCR using total RNA prepared from Con A-induced spleen cells (26). For the preparation of single-chain IL-12 and IL-27 expression vectors, fragments encoding the mature part of p40 or EBI3, followed by the (Gly4Ser)3 linker and then by the mature coding sequence of p35 or p28, respectively, were generated using standard PCR methods and cloned into the p3xFLAG-CMV-9 vector (Sigma-Aldrich). B16F10 cells were transfected with expression vectors or empty vector by using Fugene 6 (Roche) and wee selected with geneticin.
Preparation of purified rIL-27 protein
Mouse rIL-27 was prepared as a soluble tagged fusion protein by flexibly linking EBI3 to p28 as described previously (31), and human rIL-27 was also prepared similarly. The endotoxin level was <8 pg/1 μg of rIL-27 as determined by the Limulus amebocyte lysate method (Wako Pure Chemicals).
Tumor experiments
Tumor cells (105) were injected s.c. or i.v. into mice. The diameters of s.c. tumors were measured once weekly with automatic calipers. Tumor size was expressed as the square root of tumor area defined as the product of the two perpendicular diameters ((a × b)1/2). The number of metastases in lung was counted under a dissecting microscope 2–3 wk after the injection. For in vivo neutralization of IP-10, mice were i.p. injected with 200 μg of anti-mouse IP-10 (1F11) (hamster IgG) 6 h before tumor implantation (day 0) and with 100 μg on days 3, 6, 9, and 12 as reported previously (32). An equal amount of normal hamster IgG (Jackson ImmunoResearch Laboratories) was applied as a negative control. Tumor size was determined on day 14.
Immunohistochemical analysis
Two to 3 wk after i.v. injection of tumor cells, mice were sacrificed and lungs were removed and immediately fixed with 10% Formalin. Tissues were embedded in paraffin. Serial 4-μm sections were prepared from each sample and used for immunohistochemical staining. The sections were incubated with an anti-VEGF polyclonal Ab (goat IgG) (Santa Cruz Biotechnology) or anti-CD31 polyclonal Ab (goat IgG) (Santa Cruz Biotechnology). They were incubated with biotin-anti-goat IgG and then a streptavidin-peroxidase complex. As a chromogen, 3,3′-diaminobenzidine was used. The sections were counterstained with hematoxylin.
Dorsal air sac (DAS) assay
The DAS assay was performed as previously described (33). Millipore chambers were covered with filters (0.45 μm pore size). The chamber containing 5 × 106 B16F10, B16F10 + Neo, and B16F10 + IL-27 cells was implanted into air sacs created surgically by injection of an appropriate volume of air under the dorsal skin of the ICR mice. One week after the implantation of the chamber, mice were sacrificed, and the skin was carefully removed. Angiogenesis was graded using 5 angiogenesis indices of 0, 1, 2, 3, and 4 according to the method previously described (33). Grade 0 means no angiogenesis, while grade 4 indicates the most pronounced angiogenesis.
Chick embryo chorioallantoic membrane (CAM) assay
The CAM assay was performed as previously described (33). Fertilized eggs were obtained from Omiya Kakin Laboratories. Using 4-day-old chick embryos in the shell, 10 μl of various concentrations of hIL-27 in 1% methyl cellulose/0.9% NaCl (vehicle) was applied to a ring on the surface of the CAM. After 48 h of exposure at 37°C, a white fat emulsion (Mitsubishi Pharmaceutical) was injected into the CAM to clearly identify the blood vessels. Angiogenic inhibition was indicated by the presence of a 3-mm diameter avascular zone around the ring. Inhibition of angiogenesis (%) means ((number of eggs showing at least 3-mm zone of inhibition)/(number of eggs used in each experimental group)) × 100. The results were expressed as the percentage of embryos showing such inhibition.
RT-PCR
HUVEC were stimulated with mouse and human rIL-27 and human rIFN-γ for 16 h, and total RNA was extracted using a guanidine thiocyanate procedure. Total RNA was also obtained from tumor tissue 14 days after the s.c. implantation of B16F10, B16F10 + Neo, and B16F10 + IL-27 cells (105). cDNA was prepared using oligo(dT) primer and SuperScript RT (Invitrogen Life Technologies), and RT-PCR was performed using TaqDNA polymerase as described (34). The following primers were also used: human (h) hypoxanthine phosphoribosyl transferase (hHPRT) sense primer, 5′-GACCAGTCAACAGGGGACAT-3′; hHPRT antisense primer, 5′-AAGCAGATGGCCACAGAACT-3′; hWSX-1 sense primer, 5′-TGGACTTTTCCGAGGATGAC-3′; hWSX-1 antisense primer, 5′-GGAGCAGCAGCAGGTAATTC-3′; hgp130 sense primer, 5′-TGCTGATTGCAAAGCAAAAC-3′; hgp130 antisense primer, 5′-CCCACTTGCTTCTTCACTCC-3′; hIP-10 sense primer, 5′-AGAGGAACCTCCAGTCTCAGC-3′; hIP-10 antisense primer, 5′-CCTCTGTGTGGTCCATCCTT-3′; hMIG sense primer, 5′-TTAAACAATTTGCCCCAAGC-3′; hMIG antisense primer, 5′-CTGTTGTGAGTGGGATGTGG-3′; mouse (m) HPRT sense primer, 5′-GTTGGATACAGGCCAGACTTTGTTG-3′; mHPRT antisense primer, 5′-GAGGGTAGGCTGGCCTATAGGCT-3′; mIP-10 sense primer, 5′-AAGTGCTGCCGTCATTTTCT-3′; mIP-10 antisense primer, 5′-CACTGGGTAAAGGGGAGTGA-3′; mMIG sense primer, 5′-TTGGGCATCATCTTCCTGGA-3′; mMIG antisense primer, 5′-GATTCAGGGTGCTTGTTGGT-3′; single-chain mIL-27 (EBI3) sense primer, 5′-AACTCCACCAGATCCACGTC-3′; single-chain mIL-27 (p28) antisense primer, 5′-AGGGGCAGCTTCTTTTCTTC-3′.
ELISA
HUVEC were stimulated with mouse and human rIL-27 and human rIFN-γ for 48 h, and culture supernatants were collected and assayed for IP-10 and MIG production by ELISA according to the manufacturer’s instructions (R&D Systems). B16F10 transfectants were cultured, and culture supernatants were assayed for FLAG-tagged IL-27 by ELISA using anti-FLAG and biotinylated anti-IL-27 p28.
Western blotting
HUVEC were stimulated with mIL-27 (10 ng/ml), hIL-27 (10 ng/ml), and hIFN-γ (1000 U/ml) for 20 and 60 min. Cells were lysed in a lysis buffer containing protease inhibitors, and resultant cell lysates were separated on an SDS-PAGE under reducing conditions and transferred to polyvinylidene difluoride membrane (Millipore) as described previously (35). The membrane was then blocked, probed with primary Ab, and then with appropriate secondary Ab conjugated to HRP, and visualized with the ECL detection system (Amersham Biosciences) according to manufacturer’s instructions.
Statistical analysis
Data are expressed as the mean ± SD. Statistical analyses were performed using the Student t test, Welch’s t test, the Mann-Whitney U test, and Fisher’s exact probability test. Values of p (two-tailed) of 0.05 or less were considered to indicate statistically significant differences.
Results
Inhibition of s.c. growth of B16F10 tumors by IL-27
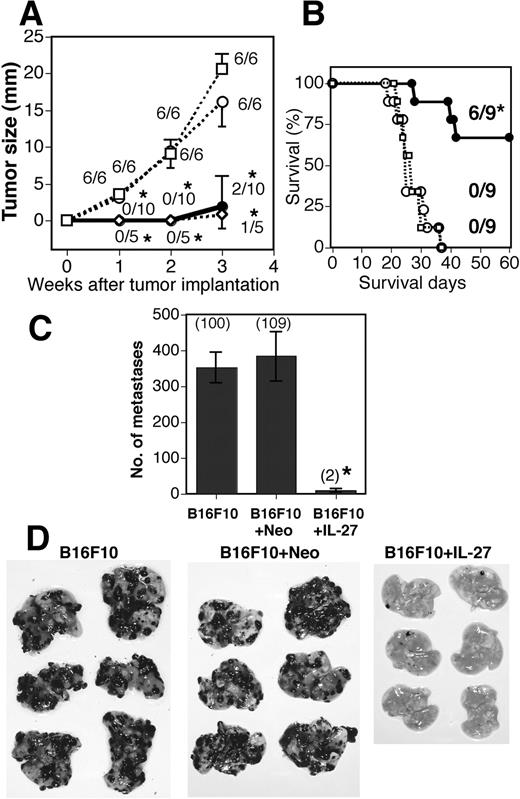
To examine the antitumor and antiangiogenic activities of IL-27 against B16F10, we first prepared murine melanoma B16F10 cells that were stably transfected with the expression vector of single-chain IL-12 (B16F10 + IL-12), IL-27 (B16F10 + IL-27), and the empty vector (B16F10 + Neo). Secretion of IL-12 and IL-27 into culture supernatants from these transfectants was confirmed by immunoprecipitation and Western blotting using anti-FLAG Ab (data not shown). The amount of IL-27 secreted from B16F10 + IL-27 cells was determined by ELISA. B16F10 + IL-27 (106) cells produced in vitro ∼5 ng of IL-27 per 24 h, while no IL-27 production was detected from B16F10 + Neo and B16F10 cells. There was no difference in cell growth in vitro and surface expression level of MHC class I among these three transfectants and parental B16F10 cells (data not shown). These tumor cells were then injected s.c. into syngenic C57BL/6 WT mice and monitored for tumor growth (Fig. 1,A) and survival (Fig. 1 B). B16F10 + IL-27 cells as well as B16F10 + IL-12 cells exhibited a strong antitumor activity as reported for colon 26 (26). Mice survived after injection with B16F10 + IL-27 cells were rechallenged with parental B16F10 or irrelevant syngenic tumor Lewis Lung carcinoma (3LL). B16F10 cells grew much more slowly in mice injected with B16F10 + IL-27 cells than in mice without injection of B16F10 + IL-27, while 3LL cells grew similarly in mice with and without injection of B16F10 + IL-27, indicating that B16F10 + IL-27 cells induce a tumor-specific protective immunity (data not shown). These results suggest that IL-27 has a strong antitumor activity against B16F10 as well as colon 26.
Inhibition of s.c. growth and pulmonary metastasis of B16F10 tumors by IL-27. WT mice were injected s.c. with B16F10 (- - -□- - -), B16F10 + Neo (- - -○- - -), B16F10 + IL-12 (- - -⋄- - -), and B16F10 + IL-27 (—–•—–) cells, and tumor growth (A) and survival (B) was monitored. Bar and asterisk indicate the SD and statistical significance compared with each control, respectively. The fraction indicates the number of tumor-bearing mice per total number of mice. ∗, 0.001 < p < 0.01 vs B16F10 + Neo and B16F10 cells by the Mann-Whitney U test (A) and Fisher’s exact probability test (B). Similar results were obtained in two independent experiments. C, Mice were injected i.v. with B16F10, B16F10 + Neo, and B16F10 + IL-27 cells. Metastasis was determined 3 wk after the injection. Data are represented as means 32 ± SD of six mice per group. Numbers in parentheses mean the percentages to B16F10 metastasis. ∗, 0.0001 < p < 0.001 vs B16F10 + Neo and B16F10 cells by Welch’s t test. Photographs of representative lungs are shown (D). Similar results were obtained in more than three independent experiments.
Inhibition of s.c. growth and pulmonary metastasis of B16F10 tumors by IL-27. WT mice were injected s.c. with B16F10 (- - -□- - -), B16F10 + Neo (- - -○- - -), B16F10 + IL-12 (- - -⋄- - -), and B16F10 + IL-27 (—–•—–) cells, and tumor growth (A) and survival (B) was monitored. Bar and asterisk indicate the SD and statistical significance compared with each control, respectively. The fraction indicates the number of tumor-bearing mice per total number of mice. ∗, 0.001 < p < 0.01 vs B16F10 + Neo and B16F10 cells by the Mann-Whitney U test (A) and Fisher’s exact probability test (B). Similar results were obtained in two independent experiments. C, Mice were injected i.v. with B16F10, B16F10 + Neo, and B16F10 + IL-27 cells. Metastasis was determined 3 wk after the injection. Data are represented as means 32 ± SD of six mice per group. Numbers in parentheses mean the percentages to B16F10 metastasis. ∗, 0.0001 < p < 0.001 vs B16F10 + Neo and B16F10 cells by Welch’s t test. Photographs of representative lungs are shown (D). Similar results were obtained in more than three independent experiments.
Inhibition of B16F10 pulmonary metastasis by IL-27
We next examined the effect of IL-27 on experimental pulmonary metastasis. Three weeks after i.v. injection of parental B16F10, B16F10 + Neo, and B16F10 + IL-27 cells, we evaluated the pulmonary metastasis (Fig. 1, C and D). Almost no pulmonary metastasis was observed in mice injected with B16F10 + IL-27, although plenty of metastases were seen in mice injected with parental B16F10 and B16F10 + Neo. The number of pulmonary metastases was markedly reduced to <2% in mice injected with B16F10 + IL-27 cells compared with B16F10 and B16F10 + Neo cells. Taken together, these results suggest that B16F10 + IL-27 cells have potent antitumor activity on s.c. tumor and experimentally induced metastasis.
Induction of antitumor and antimetastatic activities by IL-27 even in IFN-γ KO mice
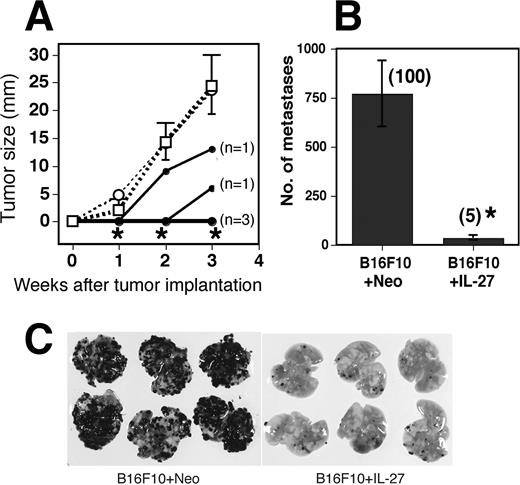
IL-27 stimulates naive CD4 + T cells to induce IFN-γ secretion in the presence of IL-12 (1, 2, 3, 4, 5). IFN-γ is well-known to induce antiangiogenic CXC chemokines such as IP-10, MIG, and IFN-inducible T cell α chemoattractant (19, 20, 21), which are important to inhibit tumor growth and metastasis. Therefore, to examine the possibility that IFN-γ induced by IL-27 might be involved in its antitumor and antimetastatic activities, we injected B16F10 + IL-27 cells into IFN-γ KO mice s.c. and i.v. and monitored tumor size and pulmonary metastasis (Fig. 2). Unexpectedly, IL-27 inhibited the growth of s.c. tumor and pulmonary metastasis in IFN-γ KO mice as in wild-type (WT) mice. These results suggest that IFN-γ is not required for the induction of antitumor and antimetastatic activities against B16F10 by IL-27.
Inhibition of s.c. growth and pulmonary metastasis by IL-27 even in IFN-γ KO mice. A, IFN-γ KO mice were injected s.c. with B16F10 (- - -□- - -), B16F10 + Neo (- - -○- - -), and B16F10 + IL-27 (—–•—–) cells and tumor growth was monitored. In the case of B16F10 + IL-27-injected mice, data of individual mice (n = 5) are shown. Tumor growth was observed in two mice from 2 and 3 wk, respectively, after the implantation, and that in three mice were not observed until 3 wk. Data in mice injected with B16F10 and B16F10 + Neo are represented as means ± SD of five mice per group. ∗, 0.001 < p < 0.01 vs B16F10 + Neo and B16F10 cells by the Mann-Whitney U test. Tumor sizes in WT mice 21 days after tumor implantation with B16F10 + Neo and B16F10 + IL-27 were 19.3 ± 3.3 (n = 3) and 1.1 ± 2.2 (n = 5), respectively. Similar results were obtained in two independent experiments. B, IFN-γ KO mice were injected i.v. with B16F10 + Neo and B16F10 + IL-27 cells and metastasis was determined 3 wk after the injection. Data are represented as means ± SD of six mice per group. Numbers in parentheses mean the percentages to B16F10 + Neo metastasis. ∗, 0.0001 < p < 0.001 vs B16F10 + Neo by Welch’s t test. Numbers of metastasis in WT mice injected with B16F10 + Neo and B16F10 + IL-27 were 320 ± 159 (n = 3) and 8 ± 5 (n = 3), respectively. Photographs of representative lungs are shown (C). Similar results were obtained in two independent experiments.
Inhibition of s.c. growth and pulmonary metastasis by IL-27 even in IFN-γ KO mice. A, IFN-γ KO mice were injected s.c. with B16F10 (- - -□- - -), B16F10 + Neo (- - -○- - -), and B16F10 + IL-27 (—–•—–) cells and tumor growth was monitored. In the case of B16F10 + IL-27-injected mice, data of individual mice (n = 5) are shown. Tumor growth was observed in two mice from 2 and 3 wk, respectively, after the implantation, and that in three mice were not observed until 3 wk. Data in mice injected with B16F10 and B16F10 + Neo are represented as means ± SD of five mice per group. ∗, 0.001 < p < 0.01 vs B16F10 + Neo and B16F10 cells by the Mann-Whitney U test. Tumor sizes in WT mice 21 days after tumor implantation with B16F10 + Neo and B16F10 + IL-27 were 19.3 ± 3.3 (n = 3) and 1.1 ± 2.2 (n = 5), respectively. Similar results were obtained in two independent experiments. B, IFN-γ KO mice were injected i.v. with B16F10 + Neo and B16F10 + IL-27 cells and metastasis was determined 3 wk after the injection. Data are represented as means ± SD of six mice per group. Numbers in parentheses mean the percentages to B16F10 + Neo metastasis. ∗, 0.0001 < p < 0.001 vs B16F10 + Neo by Welch’s t test. Numbers of metastasis in WT mice injected with B16F10 + Neo and B16F10 + IL-27 were 320 ± 159 (n = 3) and 8 ± 5 (n = 3), respectively. Photographs of representative lungs are shown (C). Similar results were obtained in two independent experiments.
Induction of antitumor and antimetastatic activities by IL-27 even in NOD-SCID mice
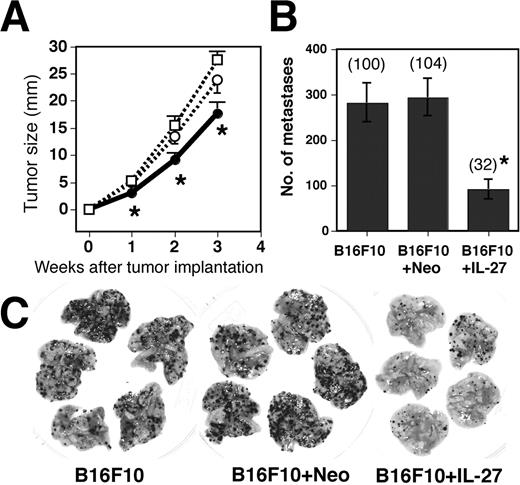
We further examined the requirement of whole host immune system for IL-27-induced antitumor activity. The NOD-SCID mouse, which lacks a functional immune system including T, B, and NK cells (30), is a good model to examine the role of the host immune system. Although the efficacy of inhibition of tumor growth and metastasis in NOD-SCID mice was not as potent as that in immunocompetent mice, IL-27 still showed a significant inhibition of tumor growth (∼25%) and metastasis (∼70%) even in NOD-SCID mice (Fig. 3). These results suggest that nonimmunological functions of the host system such as the inhibition of angiogenesis may also contribute to the induction of antitumor and antimetastatic activities against B16F10 by IL-27.
Inhibition of s.c. growth and pulmonary metastasis by IL-27 even in NOD-SCID mice. A, NOD-SCID mice were injected s.c. with B16F10 (- - -□- - -), B16F10 + Neo (- - -○- - -), and B16F10 + IL-27 (—–•—–) cells and tumor growth was monitored. Data are represented as means ± SD of five mice per group. ∗, 0.001 < p < 0.01 vs B16F10 + Neo and B16F10 cells by the Mann-Whitney U test. Similar results were obtained from two independent experiments. Tumor sizes in WT mice 21 days after tumor implantation with B16F10 + Neo and B16F10 + IL-27 were 23.7 ± 4.4 (n = 5) and 3.2 ± 4.9 (n = 6), respectively. B, NOD-SCID mice were injected i.v. with B16F10, B16F10 + Neo, and B16F10 + IL-27 cells. Metastasis was determined 2 wk after the injection. Data are represented as means ± SD of five mice per group. Numbers in parentheses mean the percentages to B16F10 metastasis. ∗, 0.0001 < p < 0.001 vs B16F10 + Neo and B16F10 cells by Student’s t test. Numbers of metastasis in WT mice injected with B16F10 + Neo and B16F10 + IL-27 were 112 (n = 2) and 49 (n = 2), respectively. Photographs of representative lungs are shown (C). Similar results were obtained in two independent experiments.
Inhibition of s.c. growth and pulmonary metastasis by IL-27 even in NOD-SCID mice. A, NOD-SCID mice were injected s.c. with B16F10 (- - -□- - -), B16F10 + Neo (- - -○- - -), and B16F10 + IL-27 (—–•—–) cells and tumor growth was monitored. Data are represented as means ± SD of five mice per group. ∗, 0.001 < p < 0.01 vs B16F10 + Neo and B16F10 cells by the Mann-Whitney U test. Similar results were obtained from two independent experiments. Tumor sizes in WT mice 21 days after tumor implantation with B16F10 + Neo and B16F10 + IL-27 were 23.7 ± 4.4 (n = 5) and 3.2 ± 4.9 (n = 6), respectively. B, NOD-SCID mice were injected i.v. with B16F10, B16F10 + Neo, and B16F10 + IL-27 cells. Metastasis was determined 2 wk after the injection. Data are represented as means ± SD of five mice per group. Numbers in parentheses mean the percentages to B16F10 metastasis. ∗, 0.0001 < p < 0.001 vs B16F10 + Neo and B16F10 cells by Student’s t test. Numbers of metastasis in WT mice injected with B16F10 + Neo and B16F10 + IL-27 were 112 (n = 2) and 49 (n = 2), respectively. Photographs of representative lungs are shown (C). Similar results were obtained in two independent experiments.
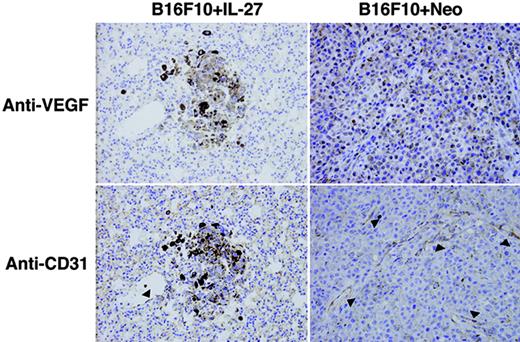
Suppression of neovascularization by IL-27 in lung metastasis
To examine the involvement of suppression of angiogenesis in the antitumor activity by IL-27, we next evaluated the inhibitory effect of IL-27 on tumor-induced neovascularization by immunohistochemical staining of each tumor tissue section with Abs against VEGF (13) and CD31 (36). Because distribution patterns of staining with anti-VEGF and anti-CD31 in tissue sections of B16F10 + IL-27 and B16F10 + Neo were largely similar among WT, IFN-γ KO, and NOD-SCID mice, only representative results in WT mice are shown in Fig. 4. Consistent with the results shown in Fig. 1, immunohistochemical analyses also revealed that the number of metastases of B16F10 + IL-27 cells was greatly lower than that of B16F10 + Neo cells (data not shown). In lungs of mice injected with B16F10 + IL-27 cells, lower and localized expression of VEGF was observed only around and within tumors, compared with those with B16F10 + Neo cells. Moreover, a decreased microvessel density within and around B16F10 + IL-27 cells as marked by arrowheads was also revealed. These results suggest that IL-27 suppresses tumor-induced neovascularization in lung metastasis.
Suppression of neovascularization by IL-27 in lung metastasis. WT mice were injected i.v. with B16F10 + IL-27 or B16F10 + Neo cells, and after 3-wk formalin-fixed lung sections of each mouse were prepared and stained with anti-VEGF or anti-CD31 using a standard avidin-biotin immunoperoxidase method. Arrowheads indicate CD31-positive vessels. Note: Dark brown particles in tumor cells are pigments from melanoma cells.
Suppression of neovascularization by IL-27 in lung metastasis. WT mice were injected i.v. with B16F10 + IL-27 or B16F10 + Neo cells, and after 3-wk formalin-fixed lung sections of each mouse were prepared and stained with anti-VEGF or anti-CD31 using a standard avidin-biotin immunoperoxidase method. Arrowheads indicate CD31-positive vessels. Note: Dark brown particles in tumor cells are pigments from melanoma cells.
Inhibition of tumor-induced angiogenesis by IL-27 in DAS assay
We then determined whether IL-27 inhibits tumor-induced angiogenesis by a mouse DAS assay (Fig. 5, A–D). A drastic induction of formation of neovessels was observed in mice transplanted with parental B16F10 and B16F10 + Neo cells-containing chambers. However, formation of such neovessels was greatly inhibited in mice transplanted with B16F10 + IL-27 cell-containing chambers, indicating that IL-27 is a powerful angiogenic inhibitor against tumor-induced angiogenesis.
Inhibition of in vivo angiogenesis by IL-27 in DAS and CAM assays. One week after implantation of the chamber containing B16F10 (A), B16F10 + Neo (B), and B16F10 + IL-27 cells (C), antiangiogenic activity of IL-27 was examined in a mouse DAS assay. Angiogenesis was judged using the index described in Materials and Methods (D). ∗, p < 0.05 vs B16F10 and ∗∗, p < 0.001versus B16F10 + Neo by Mann-Whitney U test. Data are represented as mean ± SD of five mice per group. Similar results were obtained in two independent experiments. Vehicle (1% methyl cellulose/0.9% NaCl) (E) or IL-27 (150 ng/CAM) (F) was added to the CAM surfaces of 4-day-old fertilized eggs, and the eggs were incubated for 48 h. The area surrounded by dotted curve is the place where the ring containing vehicle or IL-27 was put. IL-27 induced embryonic angiogenic inhibition and suppressed the angiogenesis dose-dependently (G). ∗, 0.001 < p < 0.01 vs control by Fisher’s exact probability test. The fraction mean number of eggs showing at least a 3-mm zone of inhibition per number of eggs used in each experimental group.
Inhibition of in vivo angiogenesis by IL-27 in DAS and CAM assays. One week after implantation of the chamber containing B16F10 (A), B16F10 + Neo (B), and B16F10 + IL-27 cells (C), antiangiogenic activity of IL-27 was examined in a mouse DAS assay. Angiogenesis was judged using the index described in Materials and Methods (D). ∗, p < 0.05 vs B16F10 and ∗∗, p < 0.001versus B16F10 + Neo by Mann-Whitney U test. Data are represented as mean ± SD of five mice per group. Similar results were obtained in two independent experiments. Vehicle (1% methyl cellulose/0.9% NaCl) (E) or IL-27 (150 ng/CAM) (F) was added to the CAM surfaces of 4-day-old fertilized eggs, and the eggs were incubated for 48 h. The area surrounded by dotted curve is the place where the ring containing vehicle or IL-27 was put. IL-27 induced embryonic angiogenic inhibition and suppressed the angiogenesis dose-dependently (G). ∗, 0.001 < p < 0.01 vs control by Fisher’s exact probability test. The fraction mean number of eggs showing at least a 3-mm zone of inhibition per number of eggs used in each experimental group.
Inhibition of embryonic angiogenesis by IL-27 in CAM assay
To study the embryonic antiangiogenic activity of IL-27, IL-27 was tested on the chick embryo CAM, which lacks mature immune system (Fig. 5, E–G). Vehicle-treated 6-day-old embryos completed formation of the blood vessel network in CAM. However, IL-27 inhibited new blood vessel growth of CAM in a dose-dependent manner as measured by the formation of an avascular zone. ED50 of IL-27 was calculated to be 165 ng/CAM. These results indicate that IL-27 is able to suppress angiogenesis in embryos. Taken together, these results in CAM and DAS assays suggest that IL-27 has a potent in vivo antiangiogenic activity, presumably resulting in suppression of tumor growth and metastasis.
Direct action of IL-27 on HUVEC, leading to the production of antiangiogenic chemokines, IP-10 and MIG
Because IFN-γ has an ability to produce chemokines such as IP-10 and MIG from HUVEC (20) and IL-27 activates STAT1 as one of its critical signaling molecules similar to IFN-γ (10), we examined whether IL-27 directly act on vein endothelial cells using HUVEC as does IFN-γ. First of all, we analyzed for expression of IL-27R subunits, WSX-1 and gp130, in HUVEC and HEK293T as well by RT-PCR (Fig. 6,A). These cells expressed both subunits, but the expression level of WSX-1 in HUVEC was less than that in HEK293T, while the expression level of gp130 was almost similar between them. We then examined whether IL-27 can induce its signaling in these cells by analyzing phosphorylation of STAT1 and STAT3 (Fig. 6,B). As expected, IFN-γ efficiently induced the phosphorylation of STAT1 but not STAT3 in both HUVEC and HEK293T cells. In contrast, mouse and human IL-27 efficiently induced the phosphorylation of both STAT1 and STAT3 in HEK293T cells, whereas less efficiently but constantly induced it in HUVEC, which appears to correlate well with the difference in mRNA expression level of WSX-1 between HEK293T cells and HUVEC. We further examined whether IL-27 can induce the production of chemokines such as IP-10 and MIG as does IFN-γ (Fig. 6, C and D). As expected, IFN-γ induced strong expression of IP-10 and MIG mRNA and their production in HUVEC. Similarly, but less efficiently, mouse and human IL-27 induced IP-10 and MIG mRNA expression and their production in HUVEC in a dose-dependent manner. These results suggest that IL-27 directly acts on HUVEC and induces the production of antiangiogenic chemokines, IP-10 and MIG, as does IFN-γ.
Direct action of IL-27 on HUVEC, leading to the production of antiangiogenic chemokines, IP-10 and MIG. A, Total RNA was isolated from HUVEC and HEK293T cells and analyzed for expression of IL-27R subunits, WSX-1 and gp130, using serial dilutions (3-fold) of cDNA templates by RT-PCR. B, HUVEC and HEK293T cells were stimulated with mIL-27, hIL-27, and hIFN-γ for 20 and 60 min. Total cell lysates were then prepared and subjected to Western blotting with anti-pY-STAT1, anti-STAT1, anti-pY-STAT3, and anti-STAT3 Abs. C, HUVEC were stimulated with mIL-27, hIL-27and hIFN-γ for 16 h. Total RNA was prepared and RT-PCR was performed. D, After HUVEC were stimulated with mIL-27, hIL-27, and hIFN-γ for 48 h, culture supernatants were collected and assayed for IP-10 and MIG production by ELISA in triplicate. ∗, p < 0.001 vs control by Student’s t test. Data are shown as the mean ± SD. Similar results were obtained in two independent experiments.
Direct action of IL-27 on HUVEC, leading to the production of antiangiogenic chemokines, IP-10 and MIG. A, Total RNA was isolated from HUVEC and HEK293T cells and analyzed for expression of IL-27R subunits, WSX-1 and gp130, using serial dilutions (3-fold) of cDNA templates by RT-PCR. B, HUVEC and HEK293T cells were stimulated with mIL-27, hIL-27, and hIFN-γ for 20 and 60 min. Total cell lysates were then prepared and subjected to Western blotting with anti-pY-STAT1, anti-STAT1, anti-pY-STAT3, and anti-STAT3 Abs. C, HUVEC were stimulated with mIL-27, hIL-27and hIFN-γ for 16 h. Total RNA was prepared and RT-PCR was performed. D, After HUVEC were stimulated with mIL-27, hIL-27, and hIFN-γ for 48 h, culture supernatants were collected and assayed for IP-10 and MIG production by ELISA in triplicate. ∗, p < 0.001 vs control by Student’s t test. Data are shown as the mean ± SD. Similar results were obtained in two independent experiments.
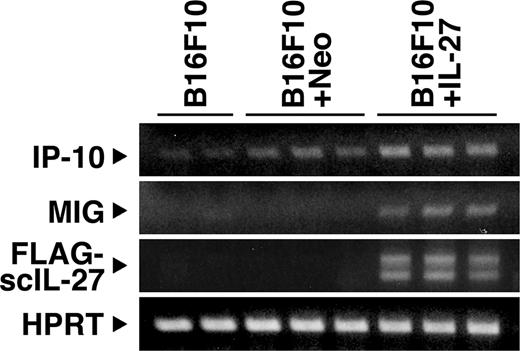
Augmented mRNA expression of IP-10 and MIG at the tumor site
Next, we examined the expression of IP-10 and MIG at the tumor site. B16F10 + IL-27, B16F19 + Neo, and B16F10 tumors were resected 14 days after implantation of 105 cells into WT mice, and total RNA was isolated and tested for IP-10 and MIG mRNA expression by RT-PCR (Fig. 7). The expression of transduced single-chain IL-27 in B16F10 + IL-27 tumor was first confirmed by RT-PCR using specific primers for single-chain IL-27. Although the expression of IP-10 was slightly detected at the tumor sites of B16F10 and B16F10 + Neo tumors, a more increased expression of IP-10 was observed at the B16F10 + IL-27 tumor site. In addition, a marked augmentation of MIG expression was more clearly observed at the B16F10 + IL-27 tumor site. These results suggest that IL-27 augments the mRNA expression of antiangiogenic IP-10 and MIG within the tumor microenvironment.
Augmented mRNA expression of IP-10 and MIG at the tumor site. Expression of IP-10 and MIG together with transduced single-chain (sc) IL-27 in the tumor site was detected by RT-PCR 14 days after tumor implantation into WT mice. The lower band for sc IL-27 may result from degradation or alternative splicing. Similar results were obtained in two independent experiments.
Augmented mRNA expression of IP-10 and MIG at the tumor site. Expression of IP-10 and MIG together with transduced single-chain (sc) IL-27 in the tumor site was detected by RT-PCR 14 days after tumor implantation into WT mice. The lower band for sc IL-27 may result from degradation or alternative splicing. Similar results were obtained in two independent experiments.
Partial but significant inhibition of IL-27-induced antitumor activity by anti-IP-10
Finally, the role of IP-10 in the antitumor activity induced by B16F10 + IL-27 cells was assessed using neutralizing mAb specific to IP-10. WT mice inoculated s.c. with B16F10 + IL-27 cells were treated with anti-IP-10 or control Ab, and tumor size was determined on day 14. The treatment with anti-IP-10 partially but significantly abrogated the antitumor effect of IL-27 (Fig. 8). This finding suggests that IP-10 plays an important role in the antitumor effect of IL-27.
Partial but significant inhibition of IL-27-induced antitumor activity by anti-IP-10. WT mice inoculated s.c. with B16F10 + IL-27 cells were injected with neutralizing mAb specific for IP-10 and control hamster IgG. Tumor size was determined 14 days after the tumor implantation. ∗1, p < 0.01 vs B16F10 + Neo and ∗2, p < 0.02 vs control IgG and by Mann-Whitney U test. Data are shown as the mean ± SD.
Partial but significant inhibition of IL-27-induced antitumor activity by anti-IP-10. WT mice inoculated s.c. with B16F10 + IL-27 cells were injected with neutralizing mAb specific for IP-10 and control hamster IgG. Tumor size was determined 14 days after the tumor implantation. ∗1, p < 0.01 vs B16F10 + Neo and ∗2, p < 0.02 vs control IgG and by Mann-Whitney U test. Data are shown as the mean ± SD.
Discussion
We showed that IL-27 has the ability to inhibit both tumor growth and metastasis of murine melanoma B16F10 not only in WT mice but also in IFN-γ KO and NOD-SCID mice (Figs. 1–3). These results suggest that nonimmunological functions of the host system such as antiangiogenic activity contribute to the induction of antitumor activity by IL-27. Immunohistochemical analyses using anti-VEGF and anti-CD31 Abs revealed that IL-27 dramatically suppresses tumor-induced neovascularization (Fig. 4). Antiangiogenic activity of IL-27 was also demonstrated in DAS and chick embryo CAM assays (Fig. 5). Moreover, IL-27 induced the production of antiangiogenic CXC chemokines, IP-10 and MIG, from HUVEC (Fig. 6) and at the tumor sites (Fig. 7). The antitumor activity of IL-27 was partially but significantly inhibited by the administration of anti-IP-10 (Fig. 8). These results indicate that IL-27 is an angiogenic inhibitor as powerful as IFN-γ, but presumably with an IFN-γ-independent mechanism. Thus, the present study provides the first and novel evidence that IL-27 has a potent antiangiogenic activity, resulting in efficient inhibition of tumor growth and metastasis, and that antiangiogenic chemokines are associated with the antitumor activity of IL-27.
Antitumor activity of IL-12 has been shown to be highly dependent on IFN-γ produced by T and NK cells in response to IL-12 (37, 38, 39). IL-18 also strongly induces production of IFN-γ in vivo (24). IFN-γ then plays an important role in enhancing Ag-specific and nonspecific immune responses, which favor tumor rejection. Moreover, IL-12 and IL-18 are known to suppress tumor growth and metastasis not only by their abilities to activate immune systems (37, 38, 39, 40, 41) but also by their antiangiogenic functions (23, 24, 25). CXC chemokines such as MIG, IP-10, and IFN-inducible T cell α chemoattractant (CXCL11), were reported to have a pivotal role in the control of angiogenesis (17, 19, 20, 21). These CXC chemokines are produced from monocytes/macrophages activated with IFN-γ, which is released by the stimulation with IL-12. These results further support that antitumor activity of IL-12 and IL-18 depends highly on IFN-γ. Because IL-27 also induces synergistic production of IFN-γ with IL-12 (37, 38, 39), it was initially expected that antitumor activity of IL-27 against B16F10 might be abrogated in IFN-γ KO mice. On the contrary, most of the antitumor activity by IL-27 is still retained even in IFN-γ KO and NOD-SCID mice (Figs. 2 and 3). In addition, a strong inhibition of neovascularization by IL-27 is also evident (Fig. 4). IL-27 suppressed new blood vessels in the chick embryos (Fig. 5, E–G). This in vivo angiostatic effect is not likely to be due to the induction of IFN-γ, since 4- to 6-day-old embryos should not have an established mature immune response. These results suggest that IFN-γ and possibly IL-12 are not essential to the induction of antimetastatic and antiangiogenic activities of IL-27 against B16F10. Instead of IFN-γ, IL-27 itself may mimic the function of IFN-γ due to the similarity in usage of JAK/STAT signaling molecules such as STAT1 (10, 42).
Because IFN-γ is not required for IL-27-induced antiangiogenic activity and endothelial cells appear to express IL-27R subunits, WSX-1 and gp130 (6), we had expected that the antiangiogenic activity by IL-27 might be attributed to direct action of IL-27 on vascular endothelial cells. Therefore, we examined the direct effect of IL-27 on HUVEC (Fig. 6). HUVEC expressed both IL-27R subunits and produced IP-10 and MIG in response to IL-27. However, suppression of in vitro proliferation of HUVEC by IL-27 could not be detected after a 3-day incubation with a high concentration (100 ng/ml) of IL-27 (data not shown). This might be because IP-10 has inhibitory effects on the proliferation of human microvascular endothelial cells (HMVEC) (43) but not of HUVEC (19), indicating the possibility of heterogeneity in the expression level of CXCR3 (44) specific for IP-10 and MIG among different endothelial cell types. In fact, HUVEC express a low level of CXCR3, while HMVEC highly express CXCR3 in a cell cycle-dependent manner (G2-M phase), and IP-10 and MIG block HMVEC proliferation in vitro (43, 45). However, the inhibitory concentration of IP-10 even in HMVEC is higher than 100 ng/ml, indicating that vascular endothelial cells are not sensitive to IP-10. Moreover, only a small percentage of vascular endothelial cells is positive for CXCR3 in normal tissues. In striking contrast, the frequency of CXCR3-positive cells in inflamed and neoplastic tissues is much higher than in normal ones (43). Furthermore, augmentation of mRNA expression of IP-10 and MIG was observed at the tumor site of B16F10 + IL-27 (Fig. 7), and the antitumor activity of IL-27 was partially but significantly reduced by treatment with anti-IP-10 (Fig. 8). Taken together, these facts suggest that antiangiogenic chemokines IP-10 and MIG that are produced by IL-27 play inhibitory roles in angiogenesis especially within tumor tissues. In contrast, because treatment with anti-IP 10 did not diminish the antitumor activity of IL-27 completely (Fig. 8), chemokines other than IP-10 or a combination of IP-10 and other chemokines including MIG may be associated with the antitumor activity of IL-27.
We (26) and others (28) have previously reported that IL-27 has potent antitumor activity, which is mediated mainly through CD8 + T cells, against murine tumor models of colon carcinoma C26 and neuroblastoma TBJ. In addition, the involvement of NK cells in the induction of antitumor activity by IL-27 has been very recently demonstrated using colon carcinoma C26 as well (27). Here, we have shown that IL-27 has the ability to induce a potent antitumor activity through another effector mechanism, antiangiogenic effect, against murine melanoma B16F10. C26 cells are highly immunogenic and express high levels of MHC class I on their cell surface. IL-27 was demonstrated to up-regulate the expression of MHC class I on TBJ cell surface (28) as reported on CD4+ T cells (10). Against these highly immunogenic tumors, IL-27 mainly uses CD8+T cells as an effector mechanism to inhibit tumor growth and metastasis. However, B16F10 is poorly immunogenic and expresses very low levels of MHC class I. Moreover, the MHC class I expression is not effectively up-regulated by IL-27, probably due to the lack of expression of one of IL-27R subunits, WSX-1, in these cells (data not shown). Against these poorly immunogenic tumors, IL-27 mainly uses antiangiogenic activity as shown in the present study and also NK cells (S. Oniki et al., unpublished data) as effector mechanisms to inhibit tumor growth and metastasis. Therefore, IL-27 has the ability to induce antitumor and antimetastatic activities through CD8+T cells, NK cells, and antiangiogenic effects, as effector mechanisms, and selectively uses these effector mechanisms, depending on the properties of tumor cells.
Although B16F10 tumor cells do not express the IL-27R subunit (WSX-1) in vitro as described above, we further examined WSX-1 expression in in vivo tumors by RT-PCR because it is still possible that the activation of cells in vivo in response to certain factors could result in induction of WSX-1. However, B16F10 + IL-27, B16F10 + Neo, and B16F10 tumors did not express detectable level of WSX-1 mRNA expression 14 days after implantation (data not shown). Thus, it is unlikely that the antitumor effects are mediated through a direct effect of IL-27 on B16F10 tumor growth or on factors secreted from B16F10 cells in this experimental setting.
Eradication of established tumors and generation of systemic antitumor immune and antimetastatic responses are necessary for cancer immunotherapy by cytokines. Among the cytokines tested, IL-12 is an effective and strong cytokine that can induce eradication of tumors and prevent metastases (46). Although a great number of promising data indicating that IL-12 could be a powerful therapeutic agent against cancer were reported in various animal models, its excessive toxicity has become a problem for its clinical application (47). In contrast, in mice treated with IL-27, we have not observed splenomegaly, liver injury with elevated serum glutamic-oxaloacetic transaminase and alanine aminotransferase activities, or intensive mononuclear cell infiltration into the liver (26), which are seen with IL-12 treatment (47). Therefore, IL-27 may be a novel attractive candidate as an agent applicable to cancer immunotherapy with potent antitumor and antimetastatic activities.
Acknowledgments
We thank the Animal Research Center of Tokyo Medical University for animal care, and Dr. J. P. Barron of the International Medical Communications Center of Tokyo Medical University for review of this manuscript.
Disclosures
M. Shimizu, M. Shimamura, J. Mizuguchi, and T. Yoshimoto are inventors on a patent with the title, “Anti-angiogenic agent with inducing ability of chemokine production.”
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This study was supported by a Grant-in-Aid for Scientific Research, “High-Tech Research Center” Project, and “University-Industry Joint Research” Project from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by a grant from the Yasuda Medical Foundation.
Abbreviations used in this paper: VEGF, vascular endothelial growth factor; IP-10, IFN-γ-inducible protein; MIG, monokine induced by IFN-γ; KO, knockout; pY, phosphotyrosine; HMVEC, human microvascular endothelial cell; h, human; DAS, dorsal air sac; CAM, chorioallantoic membrane; HPRT, hypoxanthine phosphoribosyl transferase; m, mouse; WT, wild type.